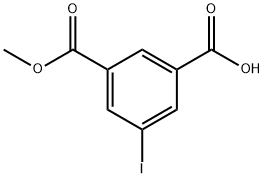
5-IODO-MONO-METHYL ISOPHTHALATE synthesis
- Product Name:5-IODO-MONO-METHYL ISOPHTHALATE
- CAS Number:93116-99-5
- Molecular formula:C9H7IO4
- Molecular Weight:306.05
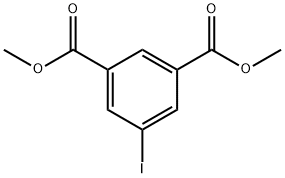
51839-15-7
140 suppliers
$6.00/250mg

93116-99-5
39 suppliers
$168.00/250mg
Yield:93116-99-5 84%
Reaction Conditions:
Stage #1: 5-iodo-isophthalic acid dimethyl esterwith methanol;sodium hydroxide in dichloromethane at 20; for 24 h;
Stage #2: with hydrogenchloride in ethyl acetate;
Steps:
73
To a solution of dimethyl 5- iodoisophthalate (12.8 g, 40 mmol), methanol (80 ml_), and CH2Cl2 (40 mL) was added NaOH (1.68 g, 42 mmol). The mixture was allowed to stir at room temperature for 24 hours. The solvents were removed under reduced pressure. Lots of white precipitate formed when water (9 mL), dichloromethane (10 mL), and ethyl acetate (10 mL) were added while stirring, which was collected by filtration, well washed with a mixture of dichloromethane (10 mL) and ethyl acetate (10 mL), and then with water (10 mL). After transferring the solid (mono sodium salt) to a separatory funnel, ethyl acetate (80 mL) and cone. HCI (3 mL) diluted with water (20 mL) were successively added. The mixture was vigorously shaken until the solid was disappeared. Then the organic layer was separated, and the aqueous layer was extracted by ethyl acetate (25 mL). The organic layers were combined and washed by brine (20 mL), dried over MgS04, filtered, and concentrated. The solid obtained was washed recrystallized from MeOH to give 10.3 g (84%) of UB-277 as a white solid. Rf = 0.33 (CH2Cl2:5%MeOH:0.1%AcOH); 1 NHMR (400 MHz, DMSO-d6) δ 13.57 (s, 1H), 8.49 - 8.27 (m, 3H), 3.89 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 165.55, 164.68, 142.24, 141.62, 133.67, 132.31, 129.36, 95.42, 53.15.
References:
WO2021/228992,2021,A1 Location in patent:Page/Page column 63
28179-47-7
106 suppliers
$140.00/250mg

93116-99-5
39 suppliers
$168.00/250mg