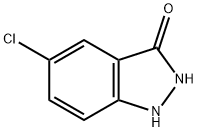
5-CHLORO-3-HYDROXY (1H)INDAZOLE synthesis
- Product Name:5-CHLORO-3-HYDROXY (1H)INDAZOLE
- CAS Number:7364-28-5
- Molecular formula:C7H5ClN2O
- Molecular Weight:168.58
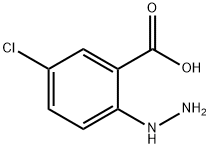
98550-70-0
1 suppliers
inquiry

7364-28-5
25 suppliers
inquiry
Yield:7364-28-5 60%
Reaction Conditions:
with hydrogenchloride in water at 100; for 3 h;Inert atmosphere;
Steps:
108.2 Step 2: preparation of 5-chloro-1H-indazol-3-ol
5-chloro-2-hydrazinylbenzoic acid (2.5 g), concentrated hydrochloric acid (10 ml) and water (200 ml) was heated at 100 °C for 3 hours. Then the mixture was concentrated to 100 mL, adjusted to pH 7.0 with sodium carbonate, filtered and dried to obtain 5-chloro-1H-indazol-3-ol (1.7 g, 60 %). 1H NMR (400 MHz, DMSO) δ 11.73 (s, 1H), 10.67 (s, 1H), 7.65 (d, J = 1.3 Hz, 1H), 7.30 (dt, J= 8.9, 5.1 Hz, 2H); MS m/z (ESI): 169 [M+H]+.
References:
EP3205650,2017,A1 Location in patent:Paragraph 0106; 0565; 0566
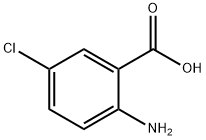
635-21-2
425 suppliers
$5.00/5g

7364-28-5
25 suppliers
inquiry