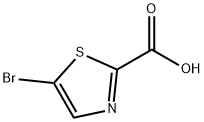
5-BROMOTHIAZOLE-2-CARBOXYLIC ACID synthesis
- Product Name:5-BROMOTHIAZOLE-2-CARBOXYLIC ACID
- CAS Number:957346-62-2
- Molecular formula:C4H2BrNO2S
- Molecular Weight:208.03
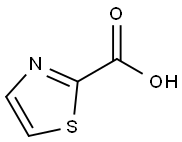
14190-59-1
191 suppliers
$15.00/1g

957346-62-2
87 suppliers
$75.00/100mg
Yield: 16%
Reaction Conditions:
Stage #1:1,3-thiazole-2-carboxylic acid with lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -78; for 0.5 h;
Stage #2: with carbon tetrabromide in tetrahydrofuran;n-heptane;ethylbenzene for 2 h;
Steps:
18.i
i) Thiazole-2-carboxylic acid (1.60 g, 12.4 mmol) was added to a solution of lithium diisopropylamine (1 M in THF/heptane/ethylbenzene, 26 mmol, 26 mL) in dry THE (100 mL) at -78 °C and the RM was stirred for 30 mi Tetrabromomethane (4.52 g, 13.6 mmol) was added and the RM was stirred for 2 h. The reactionRM was quenched by adding water (30 mL). The RM was allowed to reach RT and diluted by adding an aqueous saturated solution of NaHCO3 (50 mL). The RM was filtered through a pad of celite and extracted with EtOAc (50 mL). The organic layer was discarded and the aqueous layer acidified using a 1 N solution of HCI until pH 3-4. The solution was then extracted with EtOAc (3x 30 mL). The combined organic layer was dried over Na2SO4 and concentrated in vacuo to leave INT-18A (0.420 g, 2.02 mmol,16%). LCMS: caic. for [M+H]*=207.90, found 208.0.
References:
GRüNENTHAL GMBH;NARDI, Antonio;RATCLIFFE, Paul;CRAAN, Tobias;HERTRAMPF, Thorsten;LESCH, Bernhard;KIME, Robert;STEINHAGEN, Henning WO2015/161928, 2015, A1 Location in patent:Page/Page column 36; 53; 54