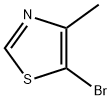
5-bromo-4-methylthiazole synthesis
- Product Name:5-bromo-4-methylthiazole
- CAS Number:111600-83-0
- Molecular formula:C4H4BrNS
- Molecular Weight:178.05
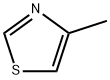
693-95-8
342 suppliers
$6.00/5g

111600-83-0
98 suppliers
$19.00/100mg
Yield: 37%
Reaction Conditions:
with bromine;acetic acid at 0 - 20;
Steps:
8
Preparation 85 -Bromo-4-methylthiazoleAdd bromine (9.27 mL, 182 mmol) to a solution of 4-methylthiazole (15.0 g, 152 mmol) in acetic acid (30 mL) at 0 0C. Slowly warm the reaction mixture to room temperature and stir overnight. Dilute with dichloromethane and wash with 1 N NaOH and brine. Dry the organic layer over sodium sulfate, filter, and concentrate under vacuum. Purify the crude product by silica gel column chromatography, elueting with hexanes/ethyl acetate (5/1) to obtain the title compound (9.94 g, 37%). 1H NMR (400 MHz, CDCl3): δ 8.69 (s, IH), 2.43 (s, 3H).
References:
ELI LILLY AND COMPANY WO2008/36579, 2008, A1 Location in patent:Page/Page column 26
20485-41-0
217 suppliers
$6.00/1g

111600-83-0
98 suppliers
$19.00/100mg