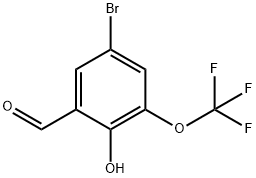
5-BROMO-2-HYDROXY-3-(TRIFLUOROMETHOXY)BENZALDEHYDE synthesis
- Product Name:5-BROMO-2-HYDROXY-3-(TRIFLUOROMETHOXY)BENZALDEHYDE
- CAS Number:497959-32-7
- Molecular formula:C8H4BrF3O3
- Molecular Weight:285.01
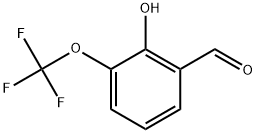
497959-31-6
69 suppliers
$38.00/250mg

497959-32-7
23 suppliers
$65.59/250mg
Yield:497959-32-7 89.5%
Reaction Conditions:
with NBS in N,N-dimethyl-formamide at 25; for 2 h;Inert atmosphere;
Steps:
53.4 53.4 Preparation of methyl 5-bromo-2-hydroxy-3-(trifluoromethoxy)benzaldehyde
To a solution of 2-hydroxy-3-(trifluoromethoxy)benzaldehyde (3.96 g, 19.2 mmol, 1 eq) in DMF (25 mL) was added NBS (3.66 g, 20.6 mmol, 1.07 eq) in one portion at 25°C under N2 atmosphere. The reaction mixture was stirred at 25°C for 2 hours. TLCpetroleum ether: ethyl acetate = 10:1 showed the reaction was completed. The reaction mixture was poured into water (80 mL) and stirred for 20 min and extracted with ethyl acetate (100 mL x 3). The combined organic phases were washed with brine (80 mL x 2), dried with anhydrous Na2SO4, filtered and concentrated under reduce pressure to give a residue. The residue was purified by column chromatography (SiO2, Eluent of 0~100% Ethyl acetate/Petroleum ether gradient) to give compound 5-bromo-2- hydroxy-3-(trifluoromethoxy)benzaldehyde (4.9 g, 17.1 mmol, 89.5% yield) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ 11.42 (s, 1 H), 10.19 (s, 1 H), 7.90 (dd, J=2.4, 1.2 Hz, 1 H), 7.83 (d, J=2.4 Hz, 1 H).
References:
WO2022/133420,2022,A1 Location in patent:Paragraph 0399; 0403
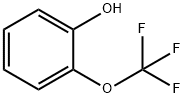
32858-93-8
235 suppliers
$10.00/1g

497959-32-7
23 suppliers
$65.59/250mg
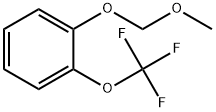
851341-36-1
20 suppliers
$391.00/5g

497959-32-7
23 suppliers
$65.59/250mg