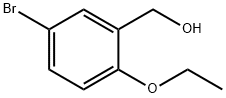
5-BROMO-2-ETHOXYBENZYL ALCOHOL synthesis
- Product Name:5-BROMO-2-ETHOXYBENZYL ALCOHOL
- CAS Number:149489-18-9
- Molecular formula:C9H11BrO2
- Molecular Weight:231.09
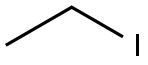
75-03-6
363 suppliers
$11.00/5g
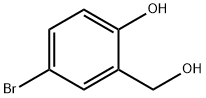
2316-64-5
160 suppliers
$9.00/1g

149489-18-9
16 suppliers
$75.00/100mg
Yield:149489-18-9 75.5 %
Reaction Conditions:
with potassium carbonate in acetonitrile at 35;
Steps:
(5-Bromo-2-ethoxyphenyl)methanol 1.633
[00350] To a solution of 4-bromo-2-(hydroxymethyl)phenol (1 g, 4.93 mmol) and iodoethane (845.80 mg, 5.42 mmol) in ACN (5 mL) was added K2CO3 (1.02 g, 7.39 mmol) at 35 °C. The mixture was stirred at 35 °C for 12 h. The reaction mixture was diluted with water (100 mb) and extracted with EA (100 mL x 3). The combined organic layer was washed with brine (100 mL x 2), dried over Na2SO4,, filtered and concentrated in vacuo. The residue was purified (PM11) to afford compound 1.633 (860 mg, 3.72 mmol, 75.5% yield) as a white solid. 1H NMR (400 MHz, CHCl3-d) δ: 7.43 (d, J = 2.4 Hz, 1H), 7.36 (dd, J = 8.8, 2.4 Hz, 1H), 6.76 (d, J = 8.8 Hz, 1H), 4.67 (s, 2H), 4.08 (q, J = 7.2 Hz, 2H), 1.46 (t, J = 7.2 Hz, 3H) ppm.
References:
WO2022/185041,2022,A1 Location in patent:Paragraph 00350
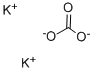
584-08-7
1200 suppliers
$5.00/100g

75-03-6
363 suppliers
$11.00/5g

2316-64-5
160 suppliers
$9.00/1g

149489-18-9
16 suppliers
$75.00/100mg