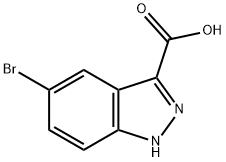
5-BROMO-1H-INDAZOLE-3-CARBOXYLIC ACID synthesis
- Product Name:5-BROMO-1H-INDAZOLE-3-CARBOXYLIC ACID
- CAS Number:1077-94-7
- Molecular formula:C8H5BrN2O2
- Molecular Weight:241.04
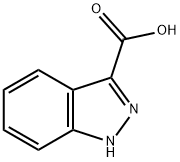
4498-67-3
492 suppliers
$6.00/5g

1077-94-7
186 suppliers
$11.00/250mg
Yield:1077-94-7 87.5%
Reaction Conditions:
with bromine;acetic acid at 90 - 120; for 16 h;
Steps:
1
Step 1
A suspension of indazole-3-carboxylic acid (CXXXI) (1.0 g, 6.16 mmol) in glacial acetic acid (60 mL) was heated at 120° C. to get a clear solution.
The solution was cooled to 90° C. A solution of bromine (0.633 mL, 12.33 mmol) in glacial acetic acid (2 mL) was added slowly to the solution while heating at 90° C.
The solution was further heated 16 h at 90° C.
The solution was cooled to room temperature, poured into ice water and further stirred at room temperature for 15 min.
The solids formed were filtered, washed with cold water and dried under vacuum at room temperature to get 5-bromo-1H-indazole-3-carboxylic acid (CXXXII) as a white solid (1.30 g, 5.39 mmol, 87.5% yield).
1H NMR (DMSO-d6) δ ppm 13.95 (s, 1H), 13.18 (br s, 1H), 8.21 (d, J=1.2 Hz, 1H), 7.65 (d, J=7.0 Hz, 1H), 7.56 (dd, J=7.0, 1.2 Hz, 1H); ESIMS found for C8H4BrN2O2 m/z 242.0 (M+H).
References:
US2015/266825,2015,A1 Location in patent:Paragraph 1359; 1360
87-48-9
293 suppliers
$10.00/5g

1077-94-7
186 suppliers
$11.00/250mg
16917-43-4
0 suppliers
inquiry

1077-94-7
186 suppliers
$11.00/250mg