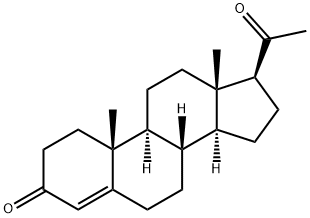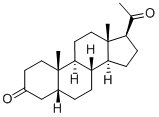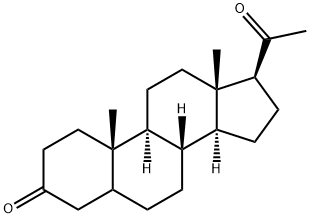
5α-Pregnane-3,20-dione synthesis
- Product Name:5α-Pregnane-3,20-dione
- CAS Number:566-65-4
- Molecular formula:C21H32O2
- Molecular Weight:316.48
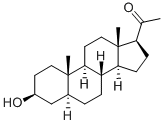
516-55-2
127 suppliers
$20.00/250mg

566-65-4
167 suppliers
$50.00/1g
Yield:566-65-4 81%
Reaction Conditions:
with sodium hypochlorite;acetic acid;sodium bromide in tetrahydrofuran at 20 - 40; for 2 h;
Steps:
2 Synthesis of (8R,10S,13S,14S,17S)-17-acetyl-10,13-dimethyltetradecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one
To a solution of the above product 1-((3S,8R,10S,13S,14S,17S)-3-hydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethenone, 3.2 g, 10 mmol) in 40 mL of THF and 10 mL of acetic acid was added NaBr (1.03 g, 0.1 eq.). It was cooled in an ice bath and was followed by the dropwise addition of NaOCl (82 mL, 10-15%, 18 eq.) at such a rate that the internal temperature was maintained <40 °C. After addition, it was stirred at room temperature for 2h. Thin layer chromatography (TLC) indicated it was complete. The mixture was diluted with dichloromethane and layers were separated. The organic layer was washed with Na2S203 (10% aq.), H20, NaHCCb (sat.) and NaCl (sat.). Drying over Na2S04 and concentration afforded 3.8 g of the crude product, which was recrystallized from CH2Cl2/Hex to give 2.57 g of the desired product (81%). NMR (400 MHz, CDC13): 2.51 (t, 1H), 2.2-2.4 (m, 3H), 2.1-2.2 (m, 1H), 2.10 (s, 3H), 1.98-2.01 (m, 2H), 1.6-1.7 (m, 4H), 1.55-1.6 (m, 1H), 1.3-1.4 (m, 7H), 1.1-1.2 (m, 2H), 0.99 (s, 3H), 0.95-0.98 (m, 1H), 0.75-0.78 (m, 1H), 0.62 (s, 3H).
References:
WO2019/209850,2019,A1 Location in patent:Paragraph 0103; 0105; 0107
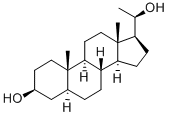
516-53-0
25 suppliers
inquiry

566-65-4
167 suppliers
$50.00/1g
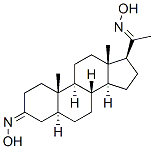
6170-12-3
0 suppliers
inquiry

566-65-4
167 suppliers
$50.00/1g
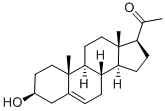
145-13-1
515 suppliers
$28.00/500mg

566-65-4
167 suppliers
$50.00/1g