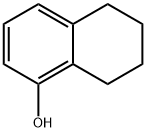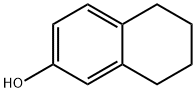
5,6,7,8-Tetrahydro-2-naphthol synthesis
- Product Name:5,6,7,8-Tetrahydro-2-naphthol
- CAS Number:1125-78-6
- Molecular formula:C10H12O
- Molecular Weight:148.2
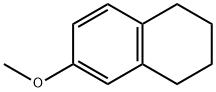
1730-48-9
66 suppliers
$44.00/5mL

1125-78-6
215 suppliers
$18.00/10g
Yield:1125-78-6 57.4%
Reaction Conditions:
Stage #1:6-methoxy-1,2,3,4-tetrahydronaphthalene with aluminum (III) chloride;thiourea at 90; for 1 h;
Stage #2: with hydrogenchloride in water;1,2-dichloro-ethane at 0 - 20;
Steps:
17
Example 17; Preparation of 5,6,7,8-tetrahydro-2-naphthol by demethylation of 5,6,7,8-tetrahydro-2- -methoxynaphthalene of the formula (VIII) CH3(VIII)To a mixture of 5.67 g (42.5 mmol) of aluminium chloride and 2.28 g (30 mmol) of thiourea 1.62 g (10 mmol) of 5,6,7,8-tetrahydro-2-methoxynaphthalene is added. The reaction mixture is heated to 90°C and maintained at this temperature for 1 hour, then cooled to room temperature, 20 ml of 1,2-dichloroethane is added and the mixture is poured onto 20 ml of 5 wt % hydrochloric acid/ice mixture.The mixture is stirred for a few minutes, then the phases are separated. The aqueous layer is extracted with 20 ml of 1,2-dichloroethane. The combined dichloroethane phases are washed with 3 x 10 ml of 5 % aqueous sodium hydroxide. The combined alkaline phases are acidified with 18 wt % hydrochloric acid solution, then extracted with 3 x 10 ml of dichloromethane. The combined dichloromethane phases are washed with water, dried over sodium sulfate and evaporated yielding 0.85 g (57.4) of 5,6,7, 8-tetrahydro-2-naphthol.
References:
RICHTER GEDEON VEGYéSZETI GYáR RT. WO2006/61666, 2006, A2 Location in patent:Page/Page column 20
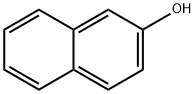
135-19-3
751 suppliers
$9.00/1g

1125-78-6
215 suppliers
$18.00/10g

135-19-3
751 suppliers
$9.00/1g

1125-78-6
215 suppliers
$18.00/10g
530-91-6
90 suppliers
$15.00/100mg

135-19-3
751 suppliers
$9.00/1g

1125-78-6
215 suppliers
$18.00/10g