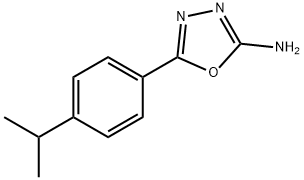
5-(4-ISOPROPYLPHENYL)-1,3,4-OXADIAZOL-2-AMINE synthesis
- Product Name:5-(4-ISOPROPYLPHENYL)-1,3,4-OXADIAZOL-2-AMINE
- CAS Number:49579-79-5
- Molecular formula:C11H13N3O
- Molecular Weight:203.24
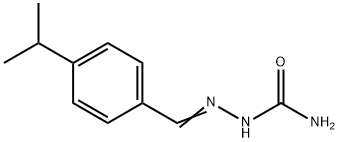
950-07-2
1 suppliers
inquiry

49579-79-5
9 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with sodium hypoiodite in water at 80 - 85;
Steps:
Synthesis of 5-substituted-1,3,4-oxadiazol-2-amines (4a-j)
General procedure: To an aqueous solution of semicarbazidehydrochloride (1:1g) and sodium acetate (8:2g),substituted benzaldehydes (1a-j) (0.02 mol) in 15 mlof ethanol were added by stirring, stirring wascontinued for another 15-30 min then the mixture waskept aside undisturbed for 1 hr and the obtainedprecipitate was collected and purified byrecrystallization from ethanol.The prepared semicarbazones (3a-j) (0.005 mol)were heated with stirring at 80-85 0 with water andsodium hypoiodate (NaOI) solution for 4-5 hr, thereaction mixture was cooled and the precipitate wascollected and recrystallized from ethanol.
References:
Joshi, Shrinivas D.;Dixit, Sheshagiri R.;More, Uttam A.;Kulkarni, Venkatrao H. [Indian Journal of Heterocyclic Chemistry,2014,vol. 24,# 2,p. 137 - 140]
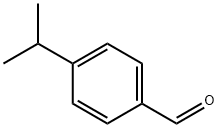
122-03-2
354 suppliers
$6.00/25g

49579-79-5
9 suppliers
$45.00/50mg