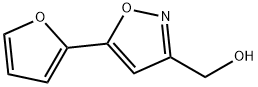
[5-(2-FURYL)ISOXAZOL-3-YL]METHANOL synthesis
- Product Name:[5-(2-FURYL)ISOXAZOL-3-YL]METHANOL
- CAS Number:852180-63-3
- Molecular formula:C8H7NO3
- Molecular Weight:165.15
33545-40-3
50 suppliers
$155.00/500mg
![[5-(2-FURYL)ISOXAZOL-3-YL]METHANOL](/CAS/GIF/852180-63-3.gif)
852180-63-3
22 suppliers
$65.00/500mg
Yield:852180-63-3 88%
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 0 - 20; for 4 h;
Steps:
3.1.1; 5.1
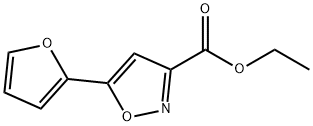
To a solution of 2.00 g of 5-furan-2-yl-isoxazole-3-carboxylic acid ethyl ester in absolute ethanol was slowly added 548 mg of sodiumborohydride at 0° C. After stirring at room temperature for 4 hrs, the reaction was quenched by addition of distilled water. The reaction solution was concentrated under reduced pressure and extracted with methylene chloride. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to produce 1.41 g of (5-furan-2-yl-isoxazol-3-yl)-methanol. (Yield 88%). This concentrate was used in the next step without further purification. NMR (acetone-d6, 200 MHz), ppm(δ): 7.82~7.78 (m, 1H), 7.05~7.00 (m, 1H), 6.71~6.63 (m, 2H), 4.71 (s, 2H)
References:
US2009/131336,2009,A1 Location in patent:Page/Page column 43