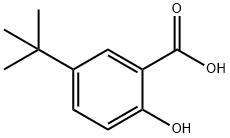
5-(1,1-dimethylethyl)salicylic acid synthesis
- Product Name:5-(1,1-dimethylethyl)salicylic acid
- CAS Number:16094-31-8
- Molecular formula:C11H14O3
- Molecular Weight:194.23
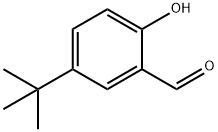
2725-53-3
147 suppliers
$20.00/1g

16094-31-8
56 suppliers
$44.00/100mg
Yield: 77.4%
Reaction Conditions:
Stage #1:2-hydroxy-5-t-butylbenzaldehyde with sodium chlorite;sodium dihydrogenphosphate;aminosulfonic acid in 1,4-dioxane;water at 0; for 1 h;
Stage #2: with sodium sulfite in 1,4-dioxane;water for 0.5 h;
Steps:
11.1
Sulfamic acid(1.76g, 18.1mmol) and sodium dihydrogenphosphate(7.33g, 47mmol) were added to a solution of 5-[(1,1-dimethyl)ethyl]-2-hydroxybenzaldehyde(2.15g, 12.1mmol) in 1,4-dioxane(100mL) and water(40mL). A solution of sodium chlorite(1.76g, 15.5mmol) in water(10mL) was added to the mixture under ice cooling, and it was stirred for 1 hour. Then, sodium sulfite(1.80g, 14.3mmol) was added to the mixture, and it was stirred for 30 minutes. Concentrated hydrochloric acid was added to the reaction mixture, and pH was adjusted to 1. The residue obtained by evaporation of 1,4-dioxane under reduced pressure was extracted with ethyl acetate. The organic layer was washed with water and brine, and dried over anhydrous magnesium sulfate. The residue obtained by evaporation of the solvent under reduced pressure was washed with n-hexane under suspension to give the title compound(1.81g, 77.4%) as a white powder.1H-NMR(DMSO-d6):δ 1.26(9H, s), 6.90(1H, d, J=9.0Hz), 7.58(1H, dd, J=8.7, 2.4Hz), 7.75(1H, d, J=2.4Hz), 11.07(1H, brs).
References:
Institute of Medicinal Molecular Design, Inc. EP1535609, 2005, A1 Location in patent:Page/Page column 74

124-38-9
129 suppliers
$175.00/23402
2198-66-5
183 suppliers
$5.00/1g

16094-31-8
56 suppliers
$44.00/100mg
1634-04-4
666 suppliers
$14.00/100g
69-72-7
1257 suppliers
$5.00/10g

16094-31-8
56 suppliers
$44.00/100mg
98-54-4
419 suppliers
$15.00/25g

16094-31-8
56 suppliers
$44.00/100mg

56-23-5
6 suppliers
$18.50/48604

98-54-4
419 suppliers
$15.00/25g

16094-31-8
56 suppliers
$44.00/100mg