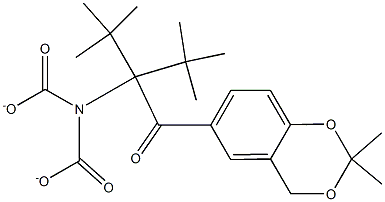
di-(tert-butyl)2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-oxoethyliMinodicarbonate synthesis
- Product Name:di-(tert-butyl)2-(2,2-diMethyl-4H-1,3-benzodioxin-6-yl)-2-oxoethyliMinodicarbonate
- CAS Number:452339-70-7
- Molecular formula:C22H31NO7
- Molecular Weight:421.48
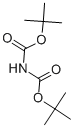
51779-32-9
290 suppliers
$5.00/1g
![2-Bromo-1-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)ethanone](/CAS2/GIF/102293-80-1.gif)
102293-80-1
87 suppliers
$265.00/250mg

452339-70-7
9 suppliers
inquiry
Yield:452339-70-7 64%
Reaction Conditions:
with Cs2CO3 in acetonitrile at 20 - 25; for 6 h;Inert atmosphere;
Steps:
4 Preparation of Di(tert-butyl) 2-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-2-oxo ethylimido dicarbonate (VI)
Example 4
Preparation of Di(tert-butyl) 2-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-2-oxo ethylimido dicarbonate (VI)
Compound V (1.0 eqt), di-t-butyl imidino dicarboxylate (1.0 eqt) and cesium carbonate (1.2 eqt) were mixed together at 20-25° C. in acetonitrile under nitrogen over a period of 6 hr.
After completion of reaction, the reaction mass was filtered off and washed with acetonitrile and concentrated under vacuum below 50° C.
The residue thus obtained was dissolved in toluene and washed with water followed by brine.
The organic fractions were dried over anhydrous sodium sulfate and were concentrated under vacuum below 60° C. to obtain the crude product.
The crude compound was slurried with diisopropyl ether, filtered and dried under vacuum at 40-45° C. to obtain the tilted compound.
Yield: 64%; purity by HPLC: 99.57%
References:
US2015/239862,2015,A1 Location in patent:Paragraph 0127; 0128
![6-Bromo-2,2-dimethyl-4H-benzo[1,3]dioxine](/CAS/20200119/GIF/52113-69-6.gif)
52113-69-6
19 suppliers
inquiry

452339-70-7
9 suppliers
inquiry
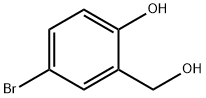
2316-64-5
161 suppliers
$9.00/1g

452339-70-7
9 suppliers
inquiry
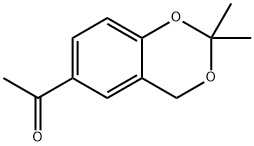
54030-34-1
5 suppliers
inquiry

452339-70-7
9 suppliers
inquiry
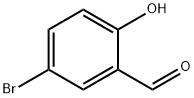
1761-61-1
384 suppliers
$5.00/5g

452339-70-7
9 suppliers
inquiry