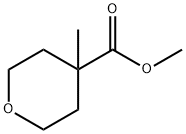
2H-Pyran-4-carboxylicacid,tetrahydro-4-methyl-,methylester(9CI) synthesis
- Product Name:2H-Pyran-4-carboxylicacid,tetrahydro-4-methyl-,methylester(9CI)
- CAS Number:443912-70-7
- Molecular formula:C8H14O3
- Molecular Weight:158.2
110238-91-0
216 suppliers
$13.00/5g

74-88-4
351 suppliers
$15.00/10g

443912-70-7
60 suppliers
$50.00/250mg
Yield:443912-70-7 100%
Reaction Conditions:
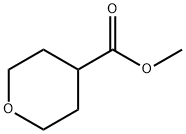
Stage #1: tetrahydropyran-4-carboxylic acid methyl esterwith n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran;hexane at -78 - 0; for 0.75 h;
Stage #2: iodomethane in tetrahydrofuran;hexane at -78 - 20;
Steps:
64.1A Methy I 4-methy ltetrahydro-2H-pyran -4-carboxy late
[1749] At -78° C., 22.1 ml ofn-butyllithium (35.4 mmol,1.6M in hexane) were slowly added dropwise to a solution of4.91 ml (35.0 mmol) of diisopropylamine in 45 ml oftetrahydrofuran,and the mixture was stirred at -78° C. for another 10min and at oo C. for another 25 min. Subsequently, at -78° C.,a solution of 5.00 g (34.7 mmol) of methyl tetrahydro-2Hpyran-4-carboxylate in 45 ml oftetrahydrofuran was added,and the mixture was stirred at -78° C. for another 15 min andat oo C. for another 30 min. At -78° C., 2.16 ml (34.7 mmol)of methyl iodide were added dropwise, and the reaction mixturewas slowly warmed to -25° C. and then to room temperatureovernight. The reaction was terminated by additionof 80 ml of O.lN hydrochloric acid, and the phases wereseparated. The aqueous phase was extracted twice with ethylacetate, the combined organic phases were dried over magnesiumsulphate and filtered and the solvent was removedunder reduced pressure. The resulting suspension was trituratedwith 20 ml of methyl tert-butyl ether, the precipitate wasfiltered off with suction and the mother liquor was concentratedunder reduced pressure, giving the title compound.Yield: 5.88 g (purity 95%, quant.)[1750] 1H-NMR (400 MHz, DMSO-d6): o [ppm]=3.70-3.64 (m, 2H), 3.64 (s, 3H), 3.37-3.30 (m, 2H), 1.94-1.88 (m,2H), 1.45-1.37 (m, 2H), 1.16 (s, 3H).
References:
US2016/52884,2016,A1 Location in patent:Paragraph 1748-1750

74-88-4
351 suppliers
$15.00/10g

443912-70-7
60 suppliers
$50.00/250mg