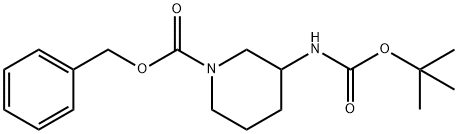
Benzyl 3-(tert-Butoxycarbonylamino)piperidine-1-carboxylate synthesis
- Product Name:Benzyl 3-(tert-Butoxycarbonylamino)piperidine-1-carboxylate
- CAS Number:406213-47-6
- Molecular formula:C18H26N2O4
- Molecular Weight:334.41
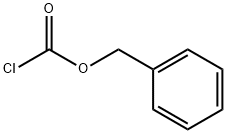
501-53-1
398 suppliers
$10.00/1g
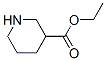
71962-74-8
49 suppliers
inquiry

406213-47-6
19 suppliers
$309.19/1g
Yield:-
Reaction Conditions:
Stage #1: benzyl chloroformate;Ethyl nipecotatewith hydrogenchloride;sodium hydroxide;diphenyl phosphoryl azide;triethylamine in ethanol;water;ethyl acetate;toluene at 0 - 20;
Stage #2: with diphenyl phosphoryl azide;triethylamine in tert-butyl alcohol at 20 - 100; for 21.5 h;
Steps:
25.a
Example 25; Piperidin-3-yl carbamic acid t-butyl ester; 25a) 3-t-Butoxycarbonylaminopiperidin-1-carboxylic acid benzyl ester; [] Benzyl chloroformate (30% solution in toluene) (88 g) was added dropwise over 30 minutes an ice-cooled a mixture of piperidin-3-carboxylic acid ethyl ester (24.3 g), triethylamine (26 mL), and ethyl acetate (300 mL). The reaction solution was filtered to remove insoluble substances, and the filtrate was filtered through a small amount of silica gel, and then concentrated. Ethanol (200 mL) and 5M aqueous sodium hydroxide solution (40 mL) were added to the residue, and this was stirred at room temperature overnight. The reaction solution was concentrated, and water (200 mL) was added to this residue, which was then extracted with t-butyl methyl ether. 5M aqueous hydrochloric acid was added to the aqueous layer, which was then extracted with ethyl acetate. The organic layer was washed with water and saturated brine, then dried over anhydrous magnesium sulfate, and concentrated to give an oily residue (30.9 g). A mixture of this residue (30 g), diphenylphosphoryl azide (24.5 mL), triethylamine (15.9 mL), and t-butanol (250 mL) was stirred at room temperature for 1.5 hours, and then in an oil bath at 100°C for 20 hours. The reaction solution was concentrated, the residue was extracted using ethyl acetate and water, and the organic layer was washed with dilute aqueous sodium bicarbonate solution and then with saturated brine. It was then dried over anhydrous magnesium sulfate, and concentrated. The residue was purified by performing silica gel column chromatography using 10% to 20% ethyl acetate/hexane, and a subsequent recrystallization using ethyl acetate and hexane to give the title compound (21.4 g).
References:
EP1568699,2005,A1 Location in patent:Page/Page column 41

501-53-1
398 suppliers
$10.00/1g

71962-74-8
49 suppliers
inquiry
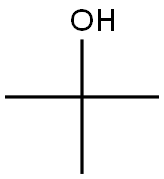
75-65-0
759 suppliers
$10.00/10ml

406213-47-6
19 suppliers
$309.19/1g
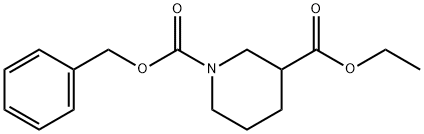
310454-53-6
84 suppliers
$15.00/1g

75-65-0
759 suppliers
$10.00/10ml

406213-47-6
19 suppliers
$309.19/1g