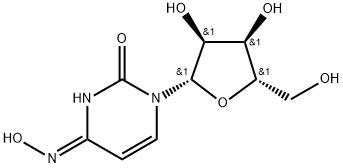
2,4(1H,3H)-Pyrimidinedione, 1-β-L-ribofuranosyl-, 4-oxime (9CI) synthesis
- Product Name:2,4(1H,3H)-Pyrimidinedione, 1-β-L-ribofuranosyl-, 4-oxime (9CI)
- CAS Number:402725-23-9
- Molecular formula:C9H13N3O6
- Molecular Weight:259.22
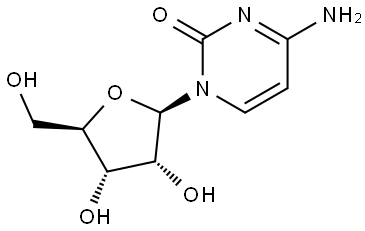
65-46-3
710 suppliers
$5.00/1g

402725-23-9
14 suppliers
inquiry
Yield:402725-23-9 25%
Reaction Conditions:
with hydroxylamine;acetic acid in water at 50; for 40 h;Sealed tube;Inert atmosphere;
Steps:
21 Example 21.EIDD-2159:
Example 21.EIDD-2159: A 2 N hydroxylamine (30.0 mL, 60.0 mmol) aqueous solution was made by adjusting a 50% w/w aq. NH2OH solution with glacial AcOH and then diluting with water to achieve the desired concentration. A sealable pressure vessel was charged with the above solution, L-cytidine (0.486 g, 2.0 mmol), and a stir bar. The vessel was sealed and the mixture was heated at 50°C for 40 h. The mixture was cooled to rt and concentrated by rotary evaporation. The crude reside was dissolved in water, and automated reverse phase flash chromatography (100 g column, gradient of 100% water to 100% MeCN) gave 300 mg of semipure material as a yellow flaky solid. The compound was taken up in MeOH and immobilized on Celite. Automated flash chromatography (12 g column, gradient of 10 to 25%) MeOH in DCM) gave -150 mg of a white flaky solid containing some occluded solvent. The residue was dissolved in water, frozen in a dry ice/acetone bath, and lyophilized to give the title compound (0.128 g, 0.494 mmol, 25% yield) as an off-white flocculent solid. Spectral analysis showed 90-95% purity; the impurity was unknown and inseparable by chromatography. 1H NMR (400 MHz, D20) δ 7.04 (d, J = 8.3 Hz, 1H), 5.83 (d, J = 5.7 Hz, 1H), 5.72 (d, J = 8.2 Hz, 1H), 4.27 (t, J = 5.5 Hz, 1H), 4.16 (t, J = 4.7 Hz, 1H), 4.03 (q, J = 3.9 Hz, 1H), 3.80 (dd, J= 12.9 Hz, 3.0 Hz, 1H), 3.72 (dd, J= 12.9 Hz, 4.2 Hz, 1H);13C NMR (100 MHz, D20) δ 151.1, 146.5, 131.2, 98.6, 87.8, 83.9, 72.4, 69.7, 60.9; HRMS calcd. forC9H14N3O6[M + H]+: 260.08771, found: 260.08734.
References:
WO2016/106050,2016,A1 Location in patent:Page/Page column 77; 78