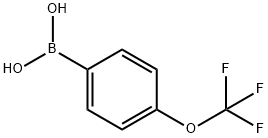
4-Trifluoromethoxyphenylboronic acid synthesis
- Product Name:4-Trifluoromethoxyphenylboronic acid
- CAS Number:139301-27-2
- Molecular formula:C7H6BF3O3
- Molecular Weight:205.93
5419-55-6
388 suppliers
$12.00/5g
407-14-7
395 suppliers
$5.00/1g

139301-27-2
393 suppliers
$14.00/5G
Yield:139301-27-2 1.306 kg (90.4%)
Reaction Conditions:
with hydrogenchloride;n-butyllithium;sodium chloride in tetrahydrofuran;n-heptane;
Steps:
1.E 4-(trifluoromethoxy)phenylboronic Acid
EXAMPLE 1E 4-(trifluoromethoxy)phenylboronic Acid A solution of 1-bromo-4-(trifluoromethoxy)benzene (1.69 kg) and triisopropyl borate (1.46 kg) in THF (6.75 L) at -70° C. was treated with 2.25 M butyllithium in hexanes (3.27 L) over 2.3 hours, stirred for 10 minutes, treated with 6M HCl (1.52 L) over 50 minutes, stirred for 18 hours at room temperature, and poured into a mixture of heptane (8.43 L) and 20% (w/w) sodium chloride (8.44 kg). This mixture was stirred for 10 minutes and separated into an aqueous fraction and an organic fraction. The organic fraction was concentrated to provide a white paste. The paste was dried under vacuum (100 mmHg) at ambient temperature with a nitrogen bleed for 2 days then at 40-50° C. for 18 hours to provide 1.306 kg (90.4%) of the desired product as a solid. 1H NMR (CDCl3, 300 MHz) δ 7.24-7.19 (m, 2H), 8.14-8.10 (m, 2H) with additional absorptions at 7.19-7.15 (m, 2H) and 8.04-8.00 (m, 2H) corresponding to the cyclic boronic acid trimer.
References:
US2002/19539,2002,A1

407-14-7
395 suppliers
$5.00/1g

139301-27-2
393 suppliers
$14.00/5G

13675-18-8
165 suppliers
$6.00/1g

407-14-7
395 suppliers
$5.00/1g

139301-27-2
393 suppliers
$14.00/5G