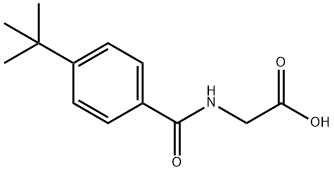
(4-TERT-BUTYL-BENZOYLAMINO)-ACETIC ACID synthesis
- Product Name:(4-TERT-BUTYL-BENZOYLAMINO)-ACETIC ACID
- CAS Number:87015-91-6
- Molecular formula:C13H17NO3
- Molecular Weight:235.28
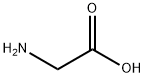
56-40-6
1245 suppliers
$5.00/25g
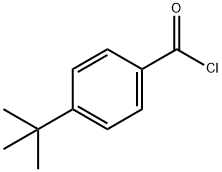
1710-98-1
150 suppliers
$12.00/5g

87015-91-6
12 suppliers
$63.61/10mgs:
Yield:87015-91-6 30%
Reaction Conditions:
Stage #1: glycinewith sodium hydroxide in water at 20;
Stage #2: p-tert-butyl benzoyl chloride in water at 60;
Steps:
4 Example 4; N-[(4-TERT-BUTYL) BENZOYLZ GLYCINE.
Glycine (0.78 g, 1.0 mmol) was dissolved in 1.0 N aqueous NAOH (10 mL) at room temperature. 4-tert-Butylbenzoyl chloride was added, and the biphasic mixture was heated to 60 C and vigorously stirred overnight. A heavy white precipitate gradually formed ; it was collected by suction filtration and washed with ether to remove 4-tert- butybenzoic acid. The product, a white powder, weighed 0. 70 g (30%) and was used without further purification.
References:
WO2004/50613,2004,A2 Location in patent:Page 57
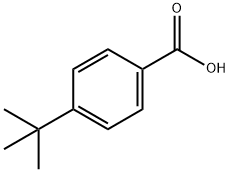
98-73-7
421 suppliers
$10.00/25g

87015-91-6
12 suppliers
$63.61/10mgs: