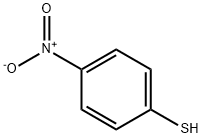
4-Nitrothiophenol synthesis
- Product Name:4-Nitrothiophenol
- CAS Number:1849-36-1
- Molecular formula:C6H5NO2S
- Molecular Weight:155.17
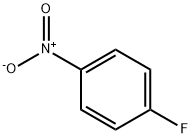
350-46-9
526 suppliers
$10.60/1gm:

1849-36-1
181 suppliers
$16.00/1g
Yield:1849-36-1 75%
Reaction Conditions:
Stage #1:4-Fluoronitrobenzene with sodium sulfide in N,N-dimethyl-formamide at 20; for 1 h;Inert atmosphere;
Stage #2: with hydrogenchloride;zinc in waterCooling with ice;Inert atmosphere;
Steps:
General Procedure
General procedure: Into an oven-dried flask equipped with a magnetic stir bar was added aryl fluoride (1.00 g, 1.0 eq.), Na2S (1.1 eq.) and DMF (5 mL) under argon. The reaction mixture was stirred at room temperature for 1 h. Then 1 M NaOH (50 mL) was added and was washed with CH2Cl2 (2 x 25 mL). The aqueous layer was acidified to pH ~ 1-2 with 6 N HCl and extracted with CH2Cl2 (2 x 50 mL). The combined organic layer was washed with brine (50 mL), dried over MgSO4, filtered and concentrated under reduced pressure to provide a crude residue. To the residue was added 10% HCl (40 mL) and cooled with an ice-water bath. Then zinc dust (4 g) was added and the mixture was stirred for 1 h. Then EtOAc (100 mL) was added and the mixture was stirred for an additional 30 minutes. The organic layer was separated and washed with water (40 mL) and brine (40 mL), dried over MgSO4, filtered and concentrated to provide the desired product with satisfactory purity.
References:
Taldone, Tony;Patel, Pallav D.;Patel, Hardik J.;Chiosis, Gabriela [Tetrahedron Letters,2012,vol. 53,# 20,p. 2548 - 2551] Location in patent:supporting information; experimental part
100-00-5
27 suppliers
$14.00/25g

1849-36-1
181 suppliers
$16.00/1g
636-98-6
30 suppliers
$17.00/5g

1849-36-1
181 suppliers
$16.00/1g
586-78-7
15 suppliers
$14.00/5g

1849-36-1
181 suppliers
$16.00/1g
100-32-3
152 suppliers
$8.00/5g

1849-36-1
181 suppliers
$16.00/1g