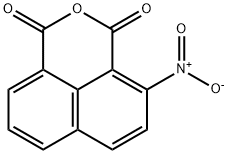
4-Nitronaphthalene-1,8-dicarboxylic anhydride synthesis
- Product Name:4-Nitronaphthalene-1,8-dicarboxylic anhydride
- CAS Number:34087-02-0
- Molecular formula:C12H5NO5
- Molecular Weight:243.17
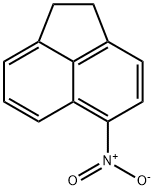
602-87-9
93 suppliers
$16.60/1g

34087-02-0
78 suppliers
inquiry
Yield: 74%
Reaction Conditions:
with sodium dichromate in acetic acid at 65 - 100; for 8.5 h;
Steps:
3 2.3 Synthesis of 4-Nitro-1, 8-naphthalic anhydride
5-Nitroacenaphthene (24.87?g, 0.125?mol) was dissolved in 248.75?mL of hot acetic acid, 158.5?g of sodium dichromate (Na2Cr2O7·2H2O) was added for 3?h?at 65-70?°C. This was warmed gradually to 98-100?°C for 30?min and further refluxed for 5?h. The contents were washed out with 0.6?L of hot water, cooled, filtered and the solid was washed with dilute HCl. It was further boiled for 30?min with 200?mL of 5% Na2CO3 solution for 30?min, filtered, the filtrate was acidified and the separated crystals dried at 120?°C for 4?h to obtain 4-nitro-1,8-naphthalic anhydride, which was recrystallised from HNO3 (d?=?1.40?g/mL) to afford colourless needles [12]. Yield?=?74%; Mp: 231-232?°C; FT-IR (KBr, cm-1) 3078, 2914 2849, 1789, 1756, 1624, 1526; 1H NMR (300?MHz, DMSO-d6): δ (ppm) 8.09 (1H, t, J?=?7.8?Hz, 3-H), 8.54 (1H, d, J?=?7.9?Hz, 8-H), 8.61 (1H, s, 2-H), 8.64 (1H, d, J?=?7.5?Hz, 9-H), 8.73 (1H, dd, J?=?8.2, 8.7?Hz, 4-H); 13C NMR (75?MHz, DMSO-d6): δ (ppm) 120.1, 122.6, 124.0, 124.3, 129.8, 130.3, 130.6, 131.1, 133.2, 149.5, 159.4, 160.0; Anal. Calcd for C12H5NO5: C, 59.27; H, 2.07; N, 5.76%; found C, 59.17; H, 2.02; N, 5.75%.
References:
Ameuru, Umar Salami;Yakubu, Mohammed Kabir;Bello, Kasali Ademola;Nkeonye, Peter Obinna;Halimehjani, Azim Ziyaei [Dyes and Pigments,2018,vol. 157,p. 190 - 197]