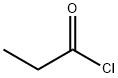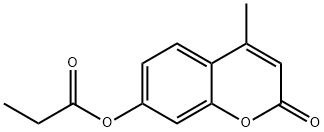
4-METHYLUMBELLIFERYL PROPIONATE synthesis
- Product Name:4-METHYLUMBELLIFERYL PROPIONATE
- CAS Number:3361-13-5
- Molecular formula:C13H12O4
- Molecular Weight:232.23
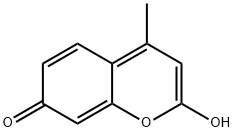
79566-13-5
6 suppliers
inquiry
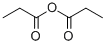
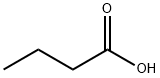
123-62-6
322 suppliers
$19.00/25g

3361-13-5
26 suppliers
$75.00/250mg
Yield:3361-13-5 97%
Reaction Conditions:
with tris(pentafluorophenyl)borate in neat (no solvent) at 20; for 0.0666667 h;Green chemistry;
Steps:
Typical experimental procedure:
General procedure: B(C6F5)3 (0.5 mol %) was added to a mixture of alcohol/phenol/thiophenol/amine (1 mmol) and acetic anhydride (1.2 mmol), and the reaction mixture was stirred at room temperature until the complete conversion of starting material (monitored by TLC). After completion of reaction, the reaction mixture was diluted with water (20 mL) and extracted with ethyl acetate (3 15 mL). The organic layer was washed with cold saturated sodium bicarbonate solution (2 20 mL) followed by brine. The organic layer was dried over MgSO4 and concentrated under reduced pressure and products were purified over silica gel column chromatography in ethylacetate/hexane. All compounds were characterized and confirmed by comparison of their spectral data and physical properties with reported literature.
References:
Prajapti, Santosh Kumar;Nagarsenkar, Atulya;Babu, Bathini Nagendra [Tetrahedron Letters,2014,vol. 55,# 10,p. 1784 - 1787]