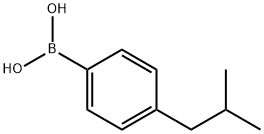
4-Isobutylphenylboronic acid synthesis
- Product Name:4-Isobutylphenylboronic acid
- CAS Number:153624-38-5
- Molecular formula:C10H15BO2
- Molecular Weight:178.04
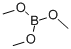
121-43-7
357 suppliers
$13.00/25mL
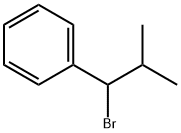
57181-82-5
11 suppliers
inquiry

153624-38-5
130 suppliers
$15.00/1g
Yield: 2.3 g (46%)
Reaction Conditions:
with hydrogenchloride;iodine;magnesium in tetrahydrofuran
Steps:
21.A A.
A. 4-isobutyl-phenylboronic acid To a suspension of 0.68 g (28.15 mmol) of magnesium turnings in 50 mL of tetrahydrofuran under argon, a crystal of iodine was added and a solution of 4-bromo-isobutylbenzene (6.0 g, 28.15 mmol) in 25 mL of tetrahydrofuran was added at such a rate that a gentle reflux was maintained. The mixture was refluxed for an additional 1 hour, cooled to room temperature and added in portions over 15 min to a solution of trimethylborate (2.93 g, 28.15 mmol) in 50 mL of ether at -78° C. under argon. After 30 min at -78° C., the solution was warmed to room temperature, stirred for 90 minutes and 10% aqueous hydrochloric acid (100 mL) was added. After 10 minutes, the solution was extracted with ether (3*100 mL) and the combined ether extracts were extracted with 1M sodium hydroxide (3*100 mL). The aqueous extracts were acidified with dilute hydrochloric acid to pH 2 and extracted with ether (3*100 mL). The combined ether extracts were washed once with water (100 mL), dried and evaporated to afford 3.5 g of a white solid. Crystallization from ether/hexanes provided 2.3 g (46%) of compound A as a white solid in two crops, m.p. 134°-135° C.
References:
Bristol-Myers Squibb Co. US5514696, 1996, A