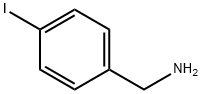
4-IODOBENZYLAMINE synthesis
- Product Name:4-IODOBENZYLAMINE
- CAS Number:39959-59-6
- Molecular formula:C7H8IN
- Molecular Weight:233.05
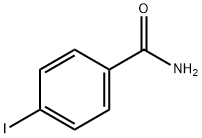
3956-07-8
83 suppliers
$27.00/1g

39959-59-6
128 suppliers
$5.00/100mg
Yield:39959-59-6 81 %
Reaction Conditions:
with 16Eu(3+)*20C5H4F3O2(1-)*8CH4O*24HO(1-)*2O(2-);pinacolborane in toluene at 110;Schlenk technique;Inert atmosphere;
Steps:
2.3 Hydroboration of amides to amine by Ln16
General procedure: The Schlenk technique was used for all the experiments.Amide (0.5 mmol, 1 equiv.), Eu16 (32 mg, 1 mol%), HBpin(290 μL, 2.0 mmol, 4 equiv.) and anhydrous toluene (1 mL)were added to the Schlenk flask under an inert atmosphere.The reaction mixture was then heated to 110 °C and stirred for 24 h. After completeness of the reaction, the mixture was cooled to room teperature and diluted with ethyl acetate(5 mL). Where after, the solution was stirred for 1 h after the addition of HCl/Et2O (83 μL of concentrated HCl dissolved in 1 mL of Et2O). The resulting white solid precipitate was separated by centrifugation and washed with copious amounts of ethyl acetate to obtain the pure ammonium salt.1H NMR and 13C NMR spectra of the ammonium salts were conducted in D2O. The hydroboration of amides by Gd16 was also performed under identical conditions.
References:
Weng, Zhen-Zhang;Chen, Chao-Long;Ye, Long-Wu;Long, La-Sheng;Zheng, Lan-Sun;Kong, Xiang-Jian [Science China Chemistry,2023,vol. 66,# 2,p. 443 - 448]
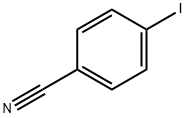
3058-39-7
282 suppliers
$10.00/1g

39959-59-6
128 suppliers
$5.00/100mg
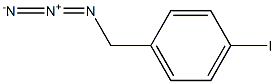
680190-94-7
1 suppliers
inquiry

39959-59-6
128 suppliers
$5.00/100mg
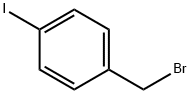
16004-15-2
307 suppliers
$5.00/1g

39959-59-6
128 suppliers
$5.00/100mg
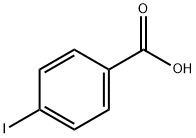
619-58-9
444 suppliers
$8.00/5g

39959-59-6
128 suppliers
$5.00/100mg