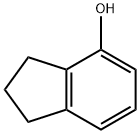
4-INDANOL synthesis
- Product Name:4-INDANOL
- CAS Number:1641-41-4
- Molecular formula:C9H10O
- Molecular Weight:134.18
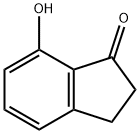
6968-35-0
112 suppliers
$17.00/250mg

1641-41-4
98 suppliers
$21.00/250mg
Yield: 90%
Reaction Conditions:
with triethylsilane in trifluoroacetic acid
Steps:
26 Tetra-n-propyl 2-6-tert-butyl-7-hydroxy-4-indanyl]ethenylidene-1,1-diphosphonate
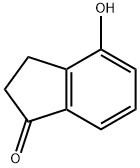
EXAMPLE 26 Tetra-n-propyl 2-6-tert-butyl-7-hydroxy-4-indanyl]ethenylidene-1,1-diphosphonate To a solution of 7-hydroxyindan-1-one (6.0 g, 40 mmole) in trifluoroacetic acid (30 ml), triethylsilane (10.23 g, 87.9 mmole) was added. The mixture was refluxed for 5 hours, poured into water (200 ml) and extracted with diethyl ether (2*200 ml). The extract was washed with water. The ethereal solution was extracted with 4NNaOH (3*50 ml). The alkaline extract was washed with diethyl ether then acidified to pH1 with hydrochloric acid. The aqueous mixture was extracted with diethyl ether and the ethereal extract was washed with water and aqueous saturated sodium chloride, was dried, and the solvents evaporated to give 4-hydroxyindane as a colourless oil (4.9 g, 90%).
References:
Symphar S.A. US5204336, 1993, A
40731-98-4
227 suppliers
$6.00/1g

1641-41-4
98 suppliers
$21.00/250mg
107300-32-3
0 suppliers
inquiry

1641-41-4
98 suppliers
$21.00/250mg
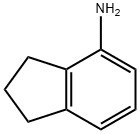
32202-61-2
105 suppliers
$29.00/1g

1641-41-4
98 suppliers
$21.00/250mg
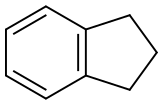
496-11-7
243 suppliers
$5.00/5g
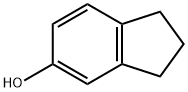
1470-94-6
142 suppliers
$21.21/5gm:

1641-41-4
98 suppliers
$21.00/250mg