4-FLUOROBENZYLMAGNESIUM CHLORIDE 0.25M& synthesis
- Product Name:4-FLUOROBENZYLMAGNESIUM CHLORIDE 0.25M&
- CAS Number:38046-82-1
- Molecular formula:C11H9BrMgO
- Molecular Weight:261.4
5111-65-9
347 suppliers
$6.00/5g

38046-82-1
36 suppliers
$248.00/50mL
Yield:-
Reaction Conditions:
with magnesium in tetrahydrofuran at 50; for 1.33333 h;
Steps:
A-11
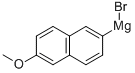
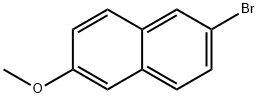
The title compound was prepared as follows: AIL, three necked rbf, equipped with a N2 inlet and a reflux condenser, was charged with Mg turnings (7.70 g, 317 mmol) and dry THF (300 mL). 6-Bromo-2-methoxy- naphthalene (compound of Formula VIII where A4 = Br and A2 = CH3) (60.0 g, 253 mmol) was added portionwise over a period of 20 min. The reaction was evacuated and placed under a N2 atm and warmed gradually to 50 °C for 1 h. In another three necked flask equipped with a N2 inlet, dropping funnel and a septum was placed 2- chlorobutyryl chloride (compound of Formula IX where R2 = CH2CH3, R3 = H, A3 = Cl, A5 = Cl) (64.0 g, 505 mmol) and dry THF (70 mL). The reaction mixture was cooled to-50 °C and the Grignard reagent as prepared above, was transferred by a cannula to the dropping funnel, under N2 pressure. The Grignard reagent was then added dropwise over 30 min. The reaction mixture was allowed to warm to room temperature (rt) and stirred for 16 h. The reaction mixture was charged with 5% HCl, the volume of THF was reduced in-vacuo, and water was added and the product was extracted with CH2C12 (3x). The combined organic layers were washed with water, brine, dried over MGS04 and concentrated in-vacuo. The crude solid was purified by silica gel chromatography (9: 1 EtOAc: Hexanes), and recrystallized from MEOH to yield the title compound. 1H NMR (CDC13, 400 MHz) 6 1. 11 (t, 3H, J=7. 2 Hz), 2.04- 2.15 (M, 1H), 2.18-2. 29 (M, 1H), 3.93 (s, 3H), 5.18-5. 22 (M, 1H), 7.16 (d, 1H, J= 2. 4 Hz), 7.22 (dd, 1H, J = 6.0, 8. 8 Hz), 7.79 (d, 1H, J = 8.8 Hz), 7.87 (d, 1H, J= 8.8 Hz), 8.02 (dd, 1H, J= 1.6, 8.8 Hz), 8.46 (s, 1H).
References:
WO2005/7631,2005,A1 Location in patent:Page/Page column 109