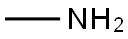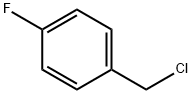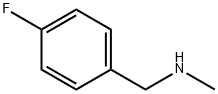
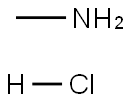
(4-FLUORO-BENZYL)-METHYL-AMINE synthesis
- Product Name:(4-FLUORO-BENZYL)-METHYL-AMINE
- CAS Number:405-66-3
- Molecular formula:C8H10FN
- Molecular Weight:139.17
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

405-66-3
150 suppliers
$13.00/1g
Yield: 96%
Reaction Conditions:
Stage #1:p-fluorobenzylamine formamide with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 64 h;
Stage #2: with sodium hydroxide;water
Steps:
114.b
b) Preparation of Intermediate (4-Fluorobenzyl) methvlamine; A solution of N- (4-fluorobenzyl) formamide (17.46 g, 114 mol) in THF (100 mL) was cooled in an ice bath (0° C). Lithium aluminium hydride (7.2 g, 190 mmol) was then added carefully in portions with stirring. The cooling bath was removed and the reaction mixture was stirred at room temperature for about 64 h. The reaction was diluted with diethyl ether and again cooled in an ice bath. It was then quenched by careful dropwise addition of aq. 1N NaOH solution followed by saturated aq. Na2SO4 solution. The resulting mixture was filtered, washing the collected solids with diethyl ether. The filtrate was concentrated to afford the title compound as a clear oil (15.2 g, 96%).
References:
PFIZER PRODUCTS INC. WO2005/80373, 2005, A1 Location in patent:Page/Page column 111
593-51-1
541 suppliers
$21.00/100g
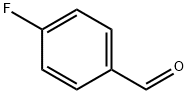
459-57-4
597 suppliers
$6.00/25g

405-66-3
150 suppliers
$13.00/1g

459-57-4
597 suppliers
$6.00/25g

405-66-3
150 suppliers
$13.00/1g
![Methanamine, N-[(4-fluorophenyl)methylene]-](/CAS/20210305/GIF/104156-56-1.gif)
104156-56-1
0 suppliers
inquiry

405-66-3
150 suppliers
$13.00/1g