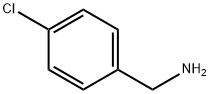
4-Chlorobenzylamine synthesis
- Product Name:4-Chlorobenzylamine
- CAS Number:104-86-9
- Molecular formula:C7H8ClN
- Molecular Weight:141.6
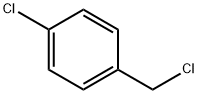
104-83-6
385 suppliers
$15.00/50g

104-86-9
291 suppliers
$6.00/5g
Yield:104-86-9 85%
Reaction Conditions:
with ammonium hydroxide;potassium dihydrogenphosphate in tert-butyl methyl ether at 20; for 0.5 h;Autoclave;Reagent/catalyst;Solvent;
Steps:
1.1-1.3; 1-2
(1) Add 127.5g (7.50mol) of liquid ammonia and 159g (0.75mol) of potassium phosphate into a 1L stainless steel autoclave;(2) Using 198g methyl tert-butyl ether to dilute 120.8g (0.75mol) parachlorobenzyl chloride, the diluted parachlorobenzyl chloride was slowly injected into the autoclave using a high-pressure liquid pump. Maintain 5mL/min, after 75min injection, use a small amount of methyl tert-butyl ether to clean the pipeline;(3) Stir at room temperature and maintain the reaction for 30 minutes. After completion, take out the reaction solution. After purification, the product p-chlorobenzylamine is obtained. The purification operation provided in this example is:The reaction solution was taken out, filtered, and the filter cake was washed twice with 50ml methyl tert-butyl ether, and the filtrate was used to recover the solvent under normal pressure, and then the product p-chlorobenzylamine could be obtained by simple distillation under reduced pressure.The p-chlorobenzylamine prepared in this example is a colorless and transparent liquid. The purity of the product as measured by gas chromatography is 99.0percent, and the yield is 85percent.
References:
Shandong Nong Pharmaceutical Xue Institute;Liu Qinsheng;Zuo Bojun;Jiang Aizhong;Chai Hongwei;Wang Minggang;Liu Jun;Li Lei;Xu Yumei;Wu Yiyang;Sun Qixia CN111470981, 2020, A Location in patent:Paragraph 00031-0045
623-03-0
475 suppliers
$6.00/25g

104-86-9
291 suppliers
$6.00/5g
3848-36-0
64 suppliers
inquiry

104-86-9
291 suppliers
$6.00/5g
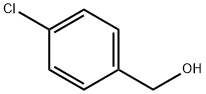
873-76-7
351 suppliers
$6.00/10g

104-86-9
291 suppliers
$6.00/5g
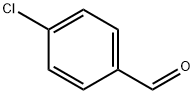
104-88-1
638 suppliers
$5.00/25g

104-86-9
291 suppliers
$6.00/5g