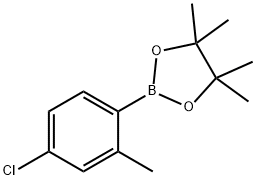
4-Chloro-2-methylphenylboronic acid pinacol ester synthesis
- Product Name:4-Chloro-2-methylphenylboronic acid pinacol ester
- CAS Number:1030832-75-7
- Molecular formula:C13H18BClO2
- Molecular Weight:252.54
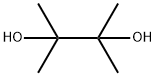
76-09-5
383 suppliers
$8.19/250mg
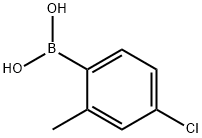
209919-30-2
179 suppliers
$8.00/1g

1030832-75-7
47 suppliers
$13.00/250mg
Yield: 97%
Reaction Conditions:
Stage #1:2,3-dimethyl-2,3-butane diol;4-chloro-2-methylphenylboronic acid in diethyl ether
Stage #2: with magnesium sulfate in diethyl ether at 20; for 24 h;
Steps:
20.1
4-Chloro-2-methylphenylboronic acid (5.Og, 29 mmol) and 100 ml of Diethyl ether were placed in round-bottom flask. Then pinacol was added (3.43, 29 mmol, leq) and the resulting reaction mixture was stirred for couple of minutes until the solution became clear. Finally, MgSO,? (6.98, 58 mmol, 2eq) was added and the solution was allowed to stir at ambient temperature, under N2 balloon overnight. After 24h the reaction was worked up. MgSO4 was filtered off and
References:
SENSORS FOR MEDICINE AND SCIENCE, INC. WO2008/66921, 2008, A2 Location in patent:Page/Page column 78-79