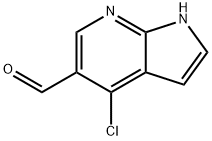
4-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-5-CARBALDEHYDE synthesis
- Product Name:4-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-5-CARBALDEHYDE
- CAS Number:958230-19-8
- Molecular formula:C8H5ClN2O
- Molecular Weight:180.59
![1H-Pyrrolo[2,3-b]pyridine-5-carboxaldehyde, 4-chloro-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/924655-39-0.gif)
924655-39-0
15 suppliers
inquiry
![4-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-5-CARBALDEHYDE](/CAS/GIF/958230-19-8.gif)
958230-19-8
119 suppliers
$28.00/100mg
Yield:958230-19-8 2.72 g
Reaction Conditions:
with trifluoroacetic acid in dichloromethane; for 24 h;
Steps:
4 REFERENCE SYNTHETIC EXAMPLE 4 4-Chloro-1H-pyrrolo[2,3-b]pyridine-5-carbaldehyde
REFERENCE SYNTHETIC EXAMPLE 4
4-Chloro-1H-pyrrolo[2,3-b]pyridine-5-carbaldehyde
A solution of 4-chloro-1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridine (5.74 g, 18.6 mmol) in tetrahydrofuran (50 mL) was cooled to -78°C, and a solution of s-butyllithium in hexane/cyclohexane (1.06 M, 27 mL, 29 mmol) was added to it, and then the mixture was stirred for 1 hour.
To the reaction mixture, N,N-dimethylformamide (7.0 mL, 90 mmol) was added, and the mixture was stirred for additional 1 hour.
The reaction mixture was mixed with 4 M hydrogen chloride in 1,4-dioxane (20 mL), followed by stirring for 30 minutes, and then mixed with water and extracted with ethyl acetate.
The organic layer was washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate and concentrated under reduced pressure.
The resulting residue was dissolved in dichloromethane (15 mL), and mixed with trifluoroacetic acid (15 mL), and the mixture was stirred for 1 day.
The reaction mixture was concentrated under reduced pressure, mixed with water and neutralized with saturated aqueous sodium hydrogencarbonate, and the precipitated solid was collected by filtration and dried under reduced pressure.
To the resulting solid, ethyl acetate (20 mL) and hexane (20 mL) were added, and the solid was collected by filtration, washed with hexane and dried under reduced pressure to obtain the title compound as a pale yellow solid (2.72 g, yield: 81%).
References:
EP2955181,2015,A1 Location in patent:Paragraph 0151; 0152
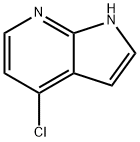
55052-28-3
487 suppliers
$6.00/1g
![4-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-5-CARBALDEHYDE](/CAS/GIF/958230-19-8.gif)
958230-19-8
119 suppliers
$28.00/100mg
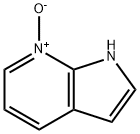
55052-24-9
150 suppliers
$80.00/5G
![4-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-5-CARBALDEHYDE](/CAS/GIF/958230-19-8.gif)
958230-19-8
119 suppliers
$28.00/100mg
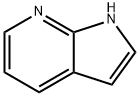
271-63-6
582 suppliers
$9.00/5g
![4-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-5-CARBALDEHYDE](/CAS/GIF/958230-19-8.gif)
958230-19-8
119 suppliers
$28.00/100mg
![1H-Pyrrolo[2,3-b]pyridine, 4-chloro-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/651744-48-8.gif)
651744-48-8
73 suppliers
$225.00/1g
![4-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE-5-CARBALDEHYDE](/CAS/GIF/958230-19-8.gif)
958230-19-8
119 suppliers
$28.00/100mg