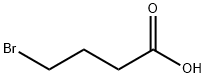
4-Bromobutyric acid synthesis
- Product Name:4-Bromobutyric acid
- CAS Number:2623-87-2
- Molecular formula:C4H7BrO2
- Molecular Weight:167
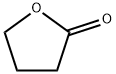
96-48-0
3 suppliers
$24.60/25g

2623-87-2
293 suppliers
$6.00/10g
Yield:2623-87-2 65%
Reaction Conditions:
with sulfuric acid;hydrogen bromide in water; for 12 h;Inert atmosphere;Reflux;
Steps:
Synthesis of 4-bromobutanoic acid (4a) and 6-bromohexanoic acid (4b)
General procedure: Typical procedure: Under a dry argon atmosphere, 48% HBr aqueous solution (41.0mL, 360mmol) and conc. sulfuric acid (9.6mL) were added dropwise to γ-butyrolactone (3a) (6.10g, 70.9mmol), and the resulting mixture was left undisturbed at room temperature for 2h. Then, after refluxing for 12h, the reaction mixture was cooled to room temperature. To this reaction mixture, 192mL of water was added, and the crude product was extracted with diethyl ether. The combined organic layer was washed with brine, dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated under reduced pressure. The residue was purified by silica gel chromatography eluted with a mixed solvent of ethyl acetate/hexane (1/2v/v). The fraction with an Rf value of 0.44 was collected and dried under reduced pressure to afford 4-bromobutanoic acid (4a) as yellow oil (7.638g, 65%). 1H NMR (400MHz, CDCl3, ppm): δ 2.19 (quint, J=6.4Hz, 2H, BrCH2CH2), 2.58 (t, J=4.2Hz, 2H, CH2COOH), and 3.48 (t, J=3.2Hz, 2H, BrCH2). 13C NMR (100MHz, CDCl3, ppm): δ 27.36 (BrCH2CH2), 32.11 (BrCH2), 32.38 (Br(CH2)2CH2), and 178.58 (COOH). IR (NaCl, cm-1): 1711 (C=O), 3046 (-OH).
References:
Ichikawa, Tsukasa;Wako, Tsuyoshi;Nemoto, Nobukatsu [Reactive and functional polymers,2016,vol. 99,p. 1 - 8]
1759-53-1
471 suppliers
$6.00/10g

2623-87-2
293 suppliers
$6.00/10g
1119-51-3
371 suppliers
$12.00/5g

2623-87-2
293 suppliers
$6.00/10g
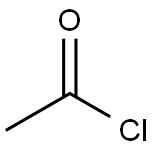
75-36-5
580 suppliers
$17.92/100G

540-51-2
245 suppliers
$17.00/5g

2623-87-2
293 suppliers
$6.00/10g
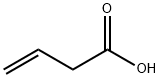
625-38-7
166 suppliers
$25.00/5g

2623-87-2
293 suppliers
$6.00/10g