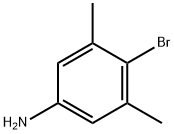
4-Bromo-3,5-dimethylaniline synthesis
- Product Name:4-Bromo-3,5-dimethylaniline
- CAS Number:59557-90-3
- Molecular formula:C8H10BrN
- Molecular Weight:200.08
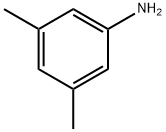
108-69-0
252 suppliers
$9.00/1g

59557-90-3
150 suppliers
$20.00/1g
Yield:59557-90-3 96%
Reaction Conditions:
Stage #1: 3,5-dimethylaminoanilinewith trifluoroacetic anhydride in dichloromethane at 0; for 0.5 h;
Stage #2: with bromine in dichloromethane at 0; for 0.0833333 h;
Steps:
205
To a solution of 3,5-dimethylaniline (3.0 ml, 24 mmol) in ice cold dichloromethane (200 ml) was added trifluoroacetic anhydride (4.18mL, 1.25 equivs). After 30 minutes, bromine (1.2 ml, 0.97 equivs) was slowly added over 5 minutes. After aqueous work-up and drying in vacuo the recovered crude (6.81 g, 96%) was taken up in dry THF (40 ml). The solution was cooled to -780C and methyl lithium'lithium bromide complex (21 ml, 1.3 equivs) was added. After 5 minutes, s-butyllithium (20 ml, 1.3 equivs) was added followed by di-t-butyldicarbonate (8.02 g, 1.6 equivs). Following aqueous work up the crude amide was taken up in a 3:1 solution of methanol: water (100 ml) followed by sodium hydroxide (5 ml, 1OM, 2 equivs). The reaction was stirred at 6O0C overnight. Solvent was removed and the crude was diluted with dichloromethane, washed with water, brine, and dried over sodium sulfate. The aniline was then taken up in dichloromethane (2 ml) and n-propylisocyanate (74 μL, 1.2 equivs). After two hours, solvent was removed and the crude residue was taken up in HCl/ethanol solution (1.2M, 15 ml) and stirred at 50°C overnight. After solvent was removed, the crude 2,6-dimethyl-4-(3- propyl-ureido)-benzoic acid was isolated as a white solid (94 mg).[0425] 1H NMR (CDCl3) δ 0.83-0.99 (m, 7H), 1.02-1.11 (m, IH), 1.46-1.77 (m, 8H), 2.11 (s, 6H), 2.48-2.57 (m, IH), 1.66-1.82 (m, 3H), 3.12-3.22 (m, 3H), 3.52-3.20 (m, 4H), 3.75-3.82- 3.90 (m, IH), 4.11-4.25 (m, IH), 5.67-5.75 (m, IH), 6.61 (s, 2H), 6.89 (d, IH, J= 6 Hz), 7.03 (s, IH), 7.22 (s, IH), 7.27-7.32 (m, IH), 7.48 (s, IH), 8.87 (br s, IH); ES-MS m/z 573 (M+H).
References:
WO2006/138350,2006,A2 Location in patent:Page/Page column 196
64835-48-9
25 suppliers
$104.00/1g

59557-90-3
150 suppliers
$20.00/1g
53906-84-6
32 suppliers
inquiry

59557-90-3
150 suppliers
$20.00/1g
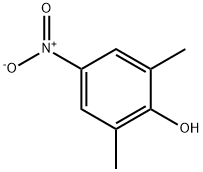
2423-71-4
112 suppliers
$7.00/250mg

59557-90-3
150 suppliers
$20.00/1g
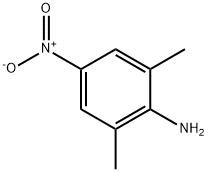
16947-63-0
66 suppliers
$85.00/50mg

59557-90-3
150 suppliers
$20.00/1g