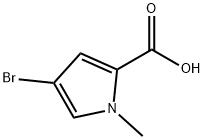
4-BROMO-1-METHYL-1H-PYRROLE-2-CARBOXYLIC ACID synthesis
- Product Name:4-BROMO-1-METHYL-1H-PYRROLE-2-CARBOXYLIC ACID
- CAS Number:875160-43-3
- Molecular formula:C6H6BrNO2
- Molecular Weight:204.02
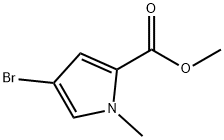
1196-90-3
95 suppliers
$22.00/100mg

875160-43-3
65 suppliers
$32.00/100mg
Yield:875160-43-3 85%
Reaction Conditions:
with methanol;sodium hydroxide at 70; for 2 h;
Steps:
5.5P.2 Step 2: 4-bromo-1-methyl-1H-pyrrole-2-carboxylic acid
To a solution of methyl 4-bromo-1-methyl-1H-pyrrole-2-carboxylate (500 mg, 2.29 mmol) in methanol (7 mL) was added 2.0 M aqueous solution of sodium hydroxide (3.0 mL). After stirring for 2 hours at 70 °C, the reaction mixture was cooled to room temperature, adjusted to pH = 3 with 2N hydrochloric acid and then extracted with ethyl acetate twice. The combined organic extracts were washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Purification via silica gel column chromatography (ethyl acetate/petroleum ether) afforded the titled compound as a yellow oil. Yield: 400 mg, 85%. LCMS (ESI) m/z = 205.9 (M+H).
References:
WO2022/109492,2022,A1 Location in patent:Page/Page column 81; 108; 109
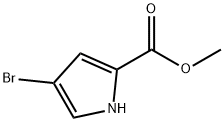
934-05-4
198 suppliers
$8.00/1g

875160-43-3
65 suppliers
$32.00/100mg