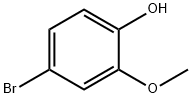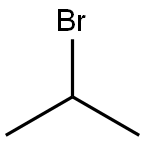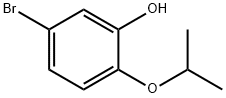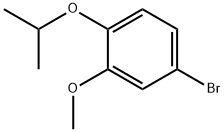
4-bromo-1-isopropoxy-2-methoxybenzene synthesis
- Product Name:4-bromo-1-isopropoxy-2-methoxybenzene
- CAS Number:138505-27-8
- Molecular formula:C10H13BrO2
- Molecular Weight:245.11
Yield:138505-27-8 100%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20 - 90;
Steps:
4.1.2. 4-(4-Isopropyloxy-3-methoxyphenyl)-3-butyn-1-ol (5b)
Isopropyl bromide (1.39 ml, 14.77 mmol) and anhydrous K2CO3 (2.04 g, 14.77 mmol) were added to a solution of 4-bromoguaiacol 4 (2.00 g, 9.85 mmol) in anhydrous DMF (10 ml). The mixture was stirred at 90 °C for 7 h, and then stirred at room temperature overnight, after which 100 ml of ethyl acetate and 100 ml of water were added. The two phases were separated and the aqueous phase was extracted again with 50 ml of ethyl acetate. The organic phases were pooled together and washed with 100 ml of HCl (0.05 M) and 100 ml of water. The organic phase was dried over anhydrous MgSO4, and the solvent was evaporated under reduced pressure. The crude compound was purified by flash column chromatography on silica gel (elution with 90/10 cyclohexane/ethyl acetate) to afford 2.43 g of isopropyl-bromoguaiacol in quantitative yield.
References:
Rivaud;Mendoza;Sauvain;Valentin;Jullian [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 15,p. 4856 - 4861] Location in patent:experimental part

67-63-0
1584 suppliers
$17.00/25ML
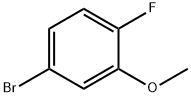
103291-07-2
126 suppliers
$10.00/5g

138505-27-8
19 suppliers
inquiry
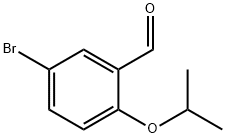
138505-25-6
49 suppliers
$58.00/250mg

138505-27-8
19 suppliers
inquiry
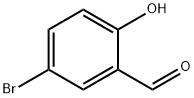
1761-61-1
382 suppliers
$5.00/5g

138505-27-8
19 suppliers
inquiry