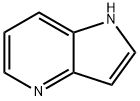
4-Azaindole synthesis
- Product Name:4-Azaindole
- CAS Number:272-49-1
- Molecular formula:C7H6N2
- Molecular Weight:118.14

947330-64-5
31 suppliers
$155.00/50mg

272-49-1
299 suppliers
$8.00/250mg
Yield:272-49-1 97%
Reaction Conditions:
with potassium tert-butylate in tetrahydrofuran;acetonitrile at 20; for 2 h;
Steps:
8.1 Preparation of 4-azaindole 1a
Product 25 7 (1.96 g, 10.3 mtnol, 1 eq.) was subjected to a cyclization reaction in the presence of a 1M 28 THF solution 29 potassium-tert-butoxide (10.3 ml, 10.3 mmol, 1 eq.) in 30 CH3CN (15 ml) at room temperature for 2 hours. The mixture was then diluted with ethyl acetate and hydrolyzed with water. After decantation, the organic phase was washed with saturated aqueous NaCl solution, dried over MgSO4 and concentrated under reduced pressure. 22 4-azaindole was isolated by flash column chromatography on silicagel (ethyl acetate/petroleum ether=1/1) with a yield of 97%
References:
CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE;UNIVERSITE D' ORLEANS;UNIVERSITE FRANCOIS RABELAIS DE TOURS;CENTRE HOSPITALIER REGIONAL UNIVERSITAIRE DE TOURS;INSERM (INSTITUT NATIONAL DE LA SANTE ET DE LA RECHERCHE MEDICALE;ROUTIER, Sylvain;SUZENET, Franck;CHALON, Sylvie;BURON, Frederic;VERCOUILLIE, Johnny;MELKI, Ronald;BOIARYNA, Liliana;GUILLOTEAU, Denis;PIERI, Laura Ronald US2019/211011, 2019, A1 Location in patent:Paragraph 0587; 0589
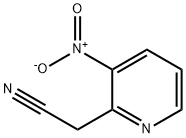
123846-65-1
24 suppliers
inquiry

272-49-1
299 suppliers
$8.00/250mg
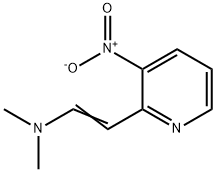
65156-92-5
5 suppliers
inquiry

272-49-1
299 suppliers
$8.00/250mg

65156-92-5
5 suppliers
inquiry

272-49-1
299 suppliers
$8.00/250mg
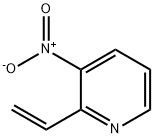
150281-83-7
13 suppliers
inquiry

272-49-1
299 suppliers
$8.00/250mg
