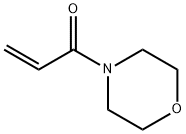
4-Acryloylmorpholine synthesis
- Product Name:4-Acryloylmorpholine
- CAS Number:5117-12-4
- Molecular formula:C7H11NO2
- Molecular Weight:141.17
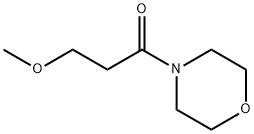
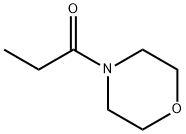
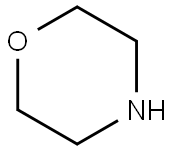
 Fig. The synthetic method 2 of 4-Acryloylmorpholine
Fig. The synthetic method 2 of 4-Acryloylmorpholine
860248-52-8
1 suppliers
inquiry

5117-12-4
282 suppliers
$6.00/25g
Yield:5117-12-4 96%
Reaction Conditions:
with sodium methoxide;4-methoxy-phenol at 200;Pyrolysis;
Steps:
10.c
(C) Into a 1000 mL four-necked flask equipped with a stirrer, a thermometer, and a gas introduction line,400 g of Meo-DEAA obtained in the step (b)6.8 g of potassium hydroxide and 0.4 g of TDA were added.Thereafter, a flask was equipped with a distillation column equipped with a packing material, a condenser and a distillation receiver, a trace nitrogen gas was flowed as a carrier,While stirring the reaction solution, the pot temperature was adjusted to 180 ° C. and the degree of vacuum was adjusted to 60 kPa,The pyrolysis reaction was carried out for 12 hours,307 g (purity = 93%, yield = 92%) of crude N, N-diethylacrylamide (DEAA) was obtained from a water condenser.Further, the obtained crude DEAA was transferred to a distillation purification apparatus equipped with a 20 cm McMahon packing (size 6 mm) packed tower and purified by distillation under reduced pressure (60 ° C./0.13 kPa)As a colorless liquid, 258 g of high purity product DEAA (purity 99.8%) was obtained. Examples 2 to 18 In Example 1,The starting materials, the catalyst, the solvent, the polymerization inhibitor and the reaction conditions of each step were changed as shown in Table 1, and synthesis was carried out in the same manner as in Example 1 to obtain the respective target compound.The results are shown in Table 2.
References:
JP2015/101554,2015,A Location in patent:Paragraph 0057; 0060
30668-14-5
30 suppliers
$100.00/1G

5117-12-4
282 suppliers
$6.00/25g
110-91-8
705 suppliers
$9.00/1g
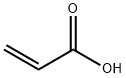
79-10-7
724 suppliers
$20.50/500ml

5117-12-4
282 suppliers
$6.00/25g
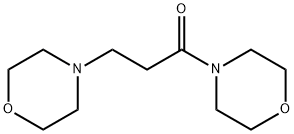
3773-84-0
0 suppliers
inquiry

5117-12-4
282 suppliers
$6.00/25g

110-91-8
705 suppliers
$9.00/1g
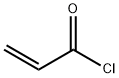
814-68-6
400 suppliers
$21.21/5gm:

5117-12-4
282 suppliers
$6.00/25g
