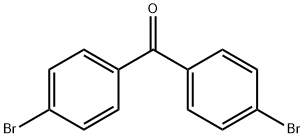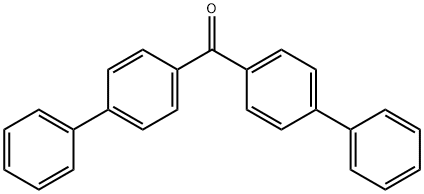
4,4'-DIPHENYLBENZOPHENONE synthesis
- Product Name:4,4'-DIPHENYLBENZOPHENONE
- CAS Number:3478-90-8
- Molecular formula:C25H18O
- Molecular Weight:334.41
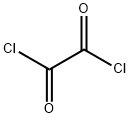
79-37-8
481 suppliers
$17.67/10gm:
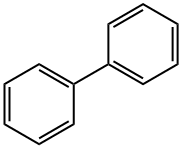
92-52-4
416 suppliers
$9.00/5g

3478-90-8
53 suppliers
$11.00/250mg
Yield: 63%
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 15 - 20;Schlenk technique;Inert atmosphere;
Steps:
4 2.2 2,2'-bis-(4,5,9,10-tetrahydropyrenyl) ketone (1)
General procedure: Aluminum chloride (2 g, 15 mmol) was introduced into a Schlenk flask and deaerated under vacuum. Dry, deaerated dichloromethane (50 mL) and then oxalyl chloride (1.7 mL, 20 mmol) were added dropwise in 20 min at 15 °C. 8mL dry and deaerated dichloromethane solution of 4,5,9,10-tetrahydropyrene (2.06 g, 10 mmol) was added into the mixture dropwise in 1h. The reaction mixture was warmed to room temperature and stirred for 1h. A second equivalent 4,5,9,10-tetrahydropyrene (2.06 g, 10 mmol) in dry and deaerated dichloromethane was introduced portionwise over 10 min. After stirring overnight, the reaction mixture was chilled in an ice/water bath, and water (40 mL) was added dropwise over 10 min. The organic layer was extracted with dichloromethane, and the combined organic layers were washed with a saturated brine solution and water, and dried over anhydrous magnesium sulfate. After filtration and solvent evaporation, the residue was purified by silica-gel column chromatography using hexane/dichloromethane as eluent. The ketone, as a white solid, was obtained in 68% yield.
References:
Zhang, Zhaoming;Zhao, Yun;Zhang, Ran;Zhang, Lifang;Cheng, Weiqin;Ni, Zhong Hai [Dyes and Pigments,2015,vol. 118,art. no. 4688,p. 95 - 101]
![[1,1'-Biphenyl]-4-methanol, α-[1,1'-biphenyl]-4-yl-](/CAS/20210305/GIF/4596-95-6.gif)
4596-95-6
1 suppliers
inquiry

3478-90-8
53 suppliers
$11.00/250mg

79-37-8
481 suppliers
$17.67/10gm:

92-52-4
416 suppliers
$9.00/5g

3478-90-8
53 suppliers
$11.00/250mg
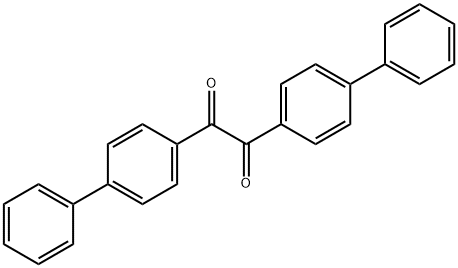
4746-80-9
18 suppliers
inquiry
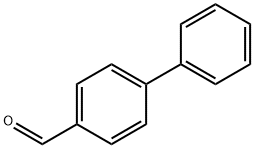
3218-36-8
346 suppliers
$6.00/5g

3478-90-8
53 suppliers
$11.00/250mg