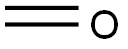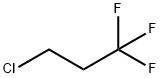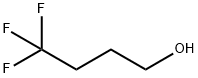
4,4,4-TRIFLUORO-1-BUTANOL synthesis
- Product Name:4,4,4-TRIFLUORO-1-BUTANOL
- CAS Number:461-18-7
- Molecular formula:C4H7F3O
- Molecular Weight:128.09
Yield:461-18-7 180 g
Reaction Conditions:
Stage #1: 3-chloro-1,1,1-trifluoropropanewith magnesium;ethylene dibromide in tetrahydrofuran at 35 - 62;
Stage #2: formaldehyd in tetrahydrofuran at 61 - 62;Grignard Reaction;Temperature;
Steps:
1-3 Example 2
At room temperature, 45.8g of magnesium chips, 170ml of tetrahydrofuran, 25g of trifluorochloropropane, 4 ml of 1,2-dibromoethane were poured into the reaction flask, the reaction was immediately initiated, the temperature in the reaction flask rose to 62 °C, the temperature was returned to 35 °C, stirred for 40min, and then 225g of trifluorochloropropane was dissolved in 500ml tetrahydrofuran solution, added dropwise to the reaction flask, kept the temperature in the reaction flask at 40-50 °C, added dropwise, raised the temperature to 45 °C and reacted for 2 hours until the magnesium chips were completely dissolved, Raise the temperature to 62 °C, add paraformaldehyde in batches, complete feeding, keep warm at 61 °C for 2 hours, complete the central control reaction, cool down to 7 °C, introduce the reaction solution into 1200ml saturated ice ammoniumchlorideaqueous solution, and then add 160ml of concentrated hydrochloric acid dropwise to adjust the pH of the solution in the reaction bottle to acidic, paraformaldehyde solid dissolved, separate the tetrahydrofuran phase, the aqueous phase is extracted with 700mlmethyltert-butylether 2 times, the organic phase is combined, and then washed once with 700ml saturated saline, sodium sulfate is dried, filtered, Tetrahydrofuran andmethyltert-butylether were distilled, the concentrated liquid was then distilled by a water pump, and the product was collected at 58 °C to obtain 180 g of colorless liquid, namely 4,4,4-trifluorobutanol, GC: 99.2%.
References:
CN115433059,2022,A Location in patent:Paragraph 0012-0014
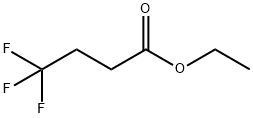
371-26-6
176 suppliers
$14.00/1g

461-18-7
213 suppliers
$7.00/1g
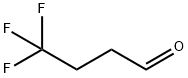
406-87-1
84 suppliers
inquiry

461-18-7
213 suppliers
$7.00/1g
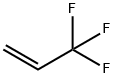
677-21-4
138 suppliers
$35.00/25g

461-18-7
213 suppliers
$7.00/1g