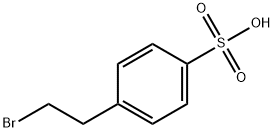
4-(2-BROMOETHYL)BENZENESULFONIC ACID synthesis
- Product Name:4-(2-BROMOETHYL)BENZENESULFONIC ACID
- CAS Number:54322-31-5
- Molecular formula:C8H9BrO3S
- Molecular Weight:265.12
103-63-9
501 suppliers
$9.00/10g

54322-31-5
46 suppliers
$210.00/1g
Yield:-
Reaction Conditions:
with chlorosulfonic acid;fuming sulphuric acid in acetonitrile at 25 - 120;
Steps:
4-(bromoethyl)benzene Sulfonic Acid
Produced According to DE3023112A1 from 2-Bromoethyl Benzene in 30% Oleum and Chlorosulfonic acid. the Resultant sulfonyl Chloride is Hydrolyzed in Water/Acetonitrile.
42 g of 30% of oleum are added to a round-bottomed flask and cooled in an ice bath.
29 g of 2-bromoethyl-benzene are dissolved in 13 g of acetonitrile and added dropwise to the oleum such that the temperature remains below 25° C.
Then it is heated in the oil bath to 40-60° C. and at this temperature 46 g of chlorosulfonic acid are added dropwise.
Subsequently it is heated for another hour to 100-120° C.
The reaction mixture is poured onto 500 ml of ice, the resulting precipitate is suctioned off, washed three times with ice-cold water, and dried. 36 g of crude product are obtained.
For purification recrystallization from 400 ml of heptane is performed.
Mixture is decanted from the brown, oily sediment.
From the supernatant, colorless crystals of the desired product are precipitated in the ice bath.
After drying 20 g are obtained.
For hydrolysis of the sulfochloride it is refluxed in a mixture of water and acetonitrile until the reaction is complete (DC-test).
The strongly acidic solution is set to a pH of 6.5 with sodium hydroxide and after concentrating 22.7 g of 4-(bromoethyl)benzene sulfonic acid sodium salt remains (mixed with formed sodium chloride).
This substance is commercially marketed as sulfonic acid or sodium sulfonate.
References:
ATTO-TEC GmbH;Kemnitzer, Norbert;Zilles, Alexander;Drexhage, Karl-Heinz;Hamers-Schneider, Monika;Arden-Jacob, Jutta US2019/100653, 2019, A1 Location in patent:Paragraph 0138-0142