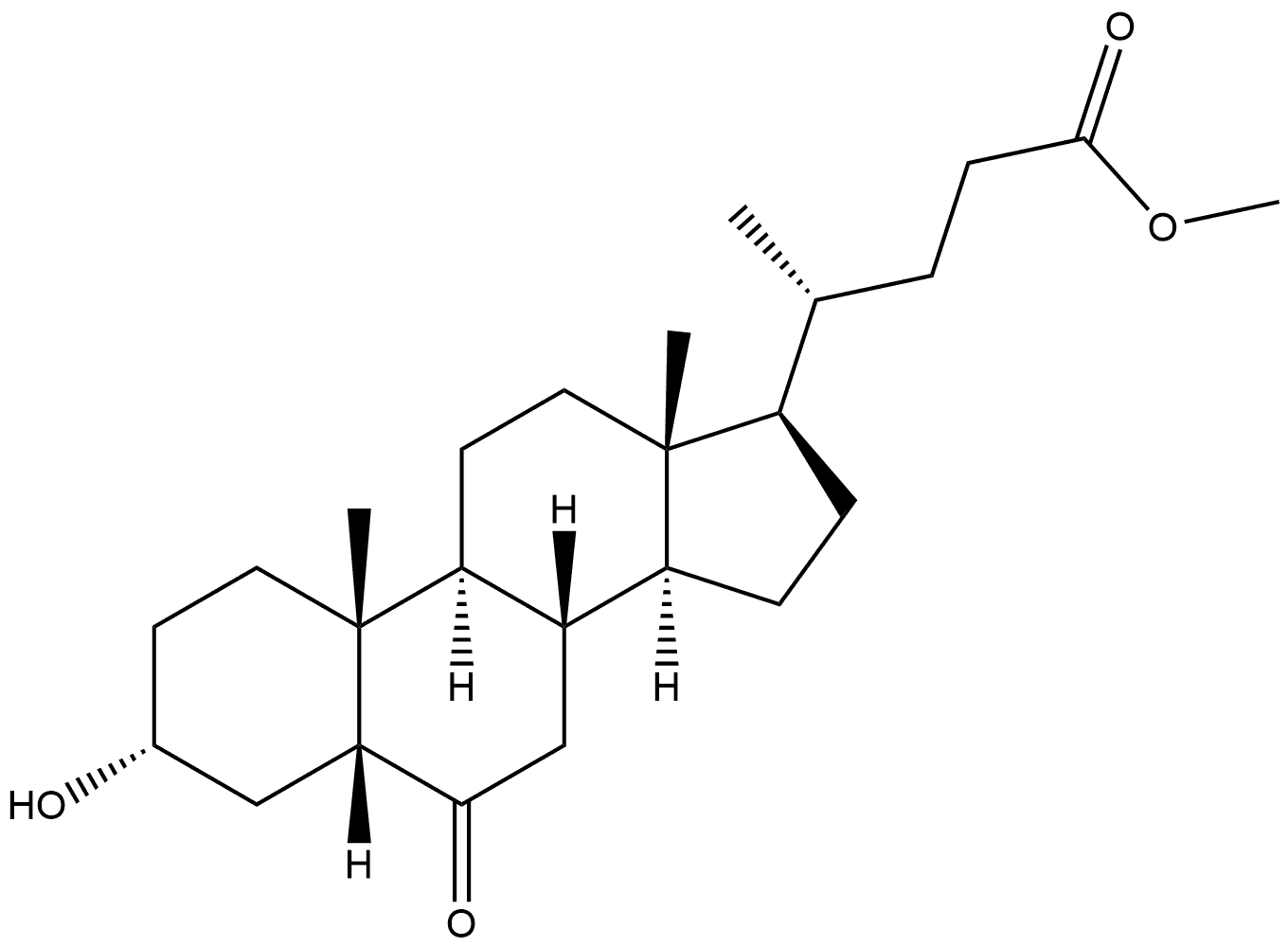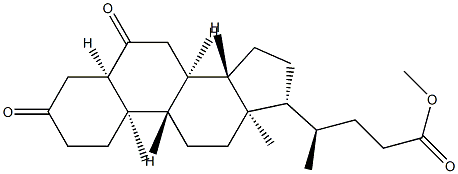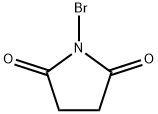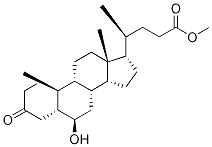
(5β,6α)-6-Hydroxy-3-oxo-cholan-24-oic Acid Methyl Ester synthesis
- Product Name:(5β,6α)-6-Hydroxy-3-oxo-cholan-24-oic Acid Methyl Ester
- CAS Number:3360-89-2
- Molecular formula:C25H40O4
- Molecular Weight:404.58
Yield:2862-62-6 85%
Reaction Conditions:
with N-Bromosuccinimide;acetic acid in water;acetone at 0 - 20; for 1.16667 h;Reagent/catalyst;
Steps:
Methyl 3α-Hydroxy-6-oxocholan-24-oate (3)
Method 1: To a stirred solution of 2 (2.40 g, 5.91 mmol) in acetone (100 mL) containing AcOH (0.40 mL, 7.09 mmol) and H2O (5 mL) was added NBS (2.10 g, 11.8 mmol) in ten portions at 0 °C. The mixture was then allowed to warm to r.t. after stirring for 10 min. The course of the reaction was monitored by TLC. After 1 h, the reaction was then quenched with sat. aq Na2SO3 (20 mL) and the mixture was evaporated under reduced pressure. EtOAc (25 mL) was added to the residue and filtered. The filtrate was washed with sat. aq Na2SO3 (2 × 25 mL), sat. aq NaHCO3 (2 × 25 mL), and brine (3 × 15 mL). The organic layer was dried (Na2SO4), filtered, and concentrated in vacuo to give the crude product, which was chromatographed on a silica gel column eluting with EtOAc-PE (1:5) to afford 3 as white crystals; yield: 2.0 g (85%). Method 2: TCCA (225 mg, 0.95 mmol) dissolved in acetone (10 mL) was added dropwise to a stirred solution of 2 (1.00 g, 2.45 mmol) in freshly distilled pyridine (0.225 mL, 2.85 mmol) and acetone (15 mL) at 0 °C, followed by gentle heating to r.t. over 40 min. The reaction was then quenched with sat. aq Na2SO3 (15 mL) and the volatiles were evaporated under reduced pressure. After extraction of the residue with EtOAc (15 mL), the separated aqueous layer was extracted with EtOAc (10 mL). The combined organic solutions were washed with aq 1 M HCl (2 × 10 mL), sat. aq NaHCO3 (3 × 25 mL) and brine (2 × 20 mL), dried (Na2SO4), and concentrated. The product was purified by silica gel column chromatography (eluent: EtOAc-PE, 1:5) to afford 3 as white crystals; yield: 753 mg (76%); mp 133-135 °C (EtOH); [α]D25 -7.1 (c 1 g/100 mL CH2Cl2). IR (KBr): 3330 (O-H), 1743 and 1690 (C=O), 1123 cm-1 (C-O). 1H NMR (400 MHz, CDCl3/TMS): δ = 0.70 (3 H, s, 18-H), 0.94 (3 H, d, J = 6.4 Hz, 21-H), 1.03 (3 H, s, 19-H), 3.69 (3 H, s), 4.13 (1 H, m, 3α-H). 13C NMR (100 MHz, CDCl3/TMS): δ = 12.04, 18.26, 21.09, 22.84, 24.15, 28.06, 30.94, 31.04, 34.36, 34.56, 35.31, 36.04, 36.21, 37.05, 37.09, 39.83, 40.27, 42.84, 50.18, 51.48, 55.94, 56.12, 67.62, 174.66, 212.71. HRMS (ESI): m/z [M + Na]+ calcd for C25H40O4Na: 427.2819; found: 427.2814.
References:
Dou, Qian;Jiang, Zhongliang [Synthesis,2016,vol. 48,# 4,art. no. SS-2015-H0518-OP,p. 588 - 594]
2868-48-6
69 suppliers
$54.60/1g

3360-89-2
6 suppliers
$165.00/25mg
83-49-8
439 suppliers
$29.00/25g

3360-89-2
6 suppliers
$165.00/25mg