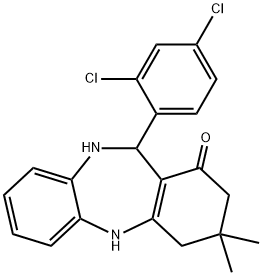
2,10-DIAZA-9-(2,4-DICHLOROPHENYL)-5,5-DIMETHYLTRICYCLO[9.4.0.0(3,8)]PENTADECA-1(11),3(8),12,14-TETRAEN-7-ONE synthesis
- Product Name:2,10-DIAZA-9-(2,4-DICHLOROPHENYL)-5,5-DIMETHYLTRICYCLO[9.4.0.0(3,8)]PENTADECA-1(11),3(8),12,14-TETRAEN-7-ONE
- CAS Number:329206-31-7
- Molecular formula:C21H20Cl2N2O
- Molecular Weight:387.3
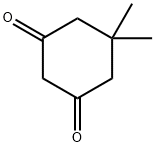
126-81-8
418 suppliers
$5.00/10g
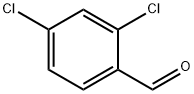
874-42-0
427 suppliers
$6.00/25g
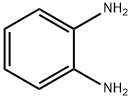
95-54-5
539 suppliers
$9.00/1g
![2,10-DIAZA-9-(2,4-DICHLOROPHENYL)-5,5-DIMETHYLTRICYCLO[9.4.0.0(3,8)]PENTADECA-1(11),3(8),12,14-TETRAEN-7-ONE](/StructureFile/ChemBookStructure2/GIF/CB7349814.gif)
329206-31-7
10 suppliers
inquiry
Yield:329206-31-7 94%
Reaction Conditions:
with 1,4-diazaniumbicyclo[2.2.2]octane diacetate at 20; for 0.25 h;Sonication;Green chemistry;Temperature;
Steps:
3. 2. General Procedure for Synthesisof Benzodiazepines
General procedure: A mixture of appropriate o-phenylendiamine (1mmol), the corresponding aldehydes (1 mmol), dimedoneand DABCO-diacetate (0.5 mmol) was placed into a Pyrexopen vessel and irradiated in a water bath under silentconditions by ultrasound (45 kHz) at room temperaturefor the required reaction times as indicated in Table 2. After completion of the reaction, as indicated by TLC, thereaction mixture was dissolved in 20 mL of H2O. Theproduct was separated by filtration, recrystallized fromEtOH and dried to afford the crystalline compounds 4a-j.The ionic liquid was recovered for subsequent use. All synthesizedcompounds were characterized by their physicalconstant, IR, 1H NMR, mass spectroscopy and elementalanalysis.
References:
Sarhandi, Shahriar;Fekri, Leila Zare;Vessally, Esmail [Acta Chimica Slovenica,2018,vol. 65,# 1,p. 246 - 252]

874-42-0
427 suppliers
$6.00/25g
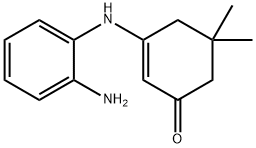
39222-69-0
29 suppliers
$203.52/1g
![2,10-DIAZA-9-(2,4-DICHLOROPHENYL)-5,5-DIMETHYLTRICYCLO[9.4.0.0(3,8)]PENTADECA-1(11),3(8),12,14-TETRAEN-7-ONE](/StructureFile/ChemBookStructure2/GIF/CB7349814.gif)
329206-31-7
10 suppliers
inquiry