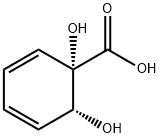
(1S,2R)-1,2-Dihydroxycyclohexa-3,5-diene-1-carboxylic acid synthesis
- Product Name:(1S,2R)-1,2-Dihydroxycyclohexa-3,5-diene-1-carboxylic acid
- CAS Number:32359-20-9
- Molecular formula:C7H8O4
- Molecular Weight:156.14
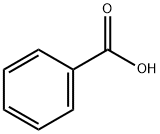
65-85-0
1398 suppliers
$10.00/25g

32359-20-9
3 suppliers
inquiry
Yield:> 99 % ee
Reaction Conditions:
Stage #1: benzoic acidwith Ralstonia eutrophus B9Enzymatic reaction;
Stage #2: with hydrogenchloride in water;
Steps:
(1S,2R)-1,2-Dihydroxycyclohexa-3,5-diene-1-carboxylic acid (12).
The whole-cell biotransformation of benzoic acid was performed based on a modified procedureestablished by Mihovilovic and co-workers.63 LB(II) medium (100 mL) was inoculated with a singlecolony of Ralstonia eutrophus B9 that was grown on LB(II) agar plates at 30 °C for two days. Theinoculated medium was incubated at 30 °C on an orbital shaker at 185 RPM until OD256 = 4.8 (1:10dilution; ~ 24 h). At this point the cellular suspension (80 mL) was used as a preculture and added to a 15L Sartorius Biostat C bioreactor that contained HMB medium (8.4 L) at pH = 7.4, aerated with sterile airat 3 L/min, agitation speed of 300 RPM, and D-fructose (50 mL of a 1.5 M aq. solution) concentration of0.009 M. The culture was grown until an OD256 = 2.8 (1:10 dilution) was achieved (~ 20 h) and theninduced with sodium benzoate (12 mL of a 1.5 M aq. solution; 18 mmol) and D-fructose (53 mL of a 1.5M aq. solution; 80 mmol). After 6 h consumption of benzoate was observed by UV analysis (265 nm) anda repetitive feeding program was initiated at which 15 min feeding of an aq. solution of sodium benzoate(22 mL, 1.5 M; 1.5 mL/min feed rate) and aq. D-fructose (22 mL, 1.5 M; 1.5 mL/min feed rate) wasperformed every 3 h over the course of 4 days; a total of approximately 170 g of sodium benzoate was fed.After the feeding regime was completed the broth was drained and separated from cell matter bycentrifugation at 5 °C (10,000 RPM). The dark brown ferment broth was concentrated in vacuo at 35 °Cto dryness to obtain several lots of ipso diene diol carboxylate as the mixed potassium/sodium salts, wascontaminated with other inorganic salts. NMR spectroscopy assay was used to establish a weight-weightpercentage of the crude material with an internal standard (potassium benzoate). A total mass of 261.5 gof crude material was obtained and corresponded to 177 g of the salt of ipso diol 12 that could be stored atroom temperature without any observable degradation (several months). 1H NMR (600 MHz, D2O) δ 6.03(dd, J = 9.5, 5.2 Hz, 1H), 5.93 - 5.81 (m, 1H), 5.71 - 5.64 (m, 2H), 4.77 (s, 1H).75 The free acid 12 wasobtained by the acidification of a cold aqueous solution of the salts with 6M HCl and extraction withEtOAc.12: 1H NMR (300 MHz, D2O) δ 6.12 (dd, J = 9.3, 5.1 Hz, 1H), 6.05 - 5.90 (m, 1H), 5.77 (d, J = 9.6 Hz,2H), 4.86 (s, 1H).
References:
Adams, David R.;Van Kempen, Johannes;Hudlicky, Jason R.;Hudlicky, Tomas [Heterocycles,2014,vol. 8,# 2,p. 1255 - 1274]
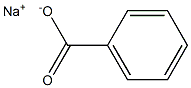
532-32-1
1325 suppliers
$5.00/25g

32359-20-9
3 suppliers
inquiry