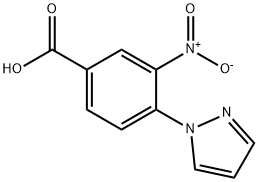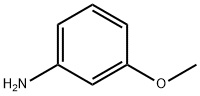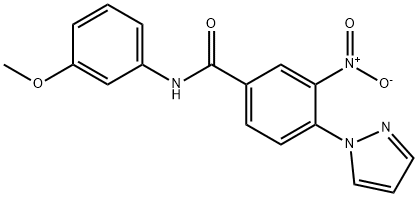
N-(3-METHOXYPHENYL)-3-NITRO-4-(1H-PYRAZOL-1-YL)BENZENECARBOXAMIDE synthesis
- Product Name:N-(3-METHOXYPHENYL)-3-NITRO-4-(1H-PYRAZOL-1-YL)BENZENECARBOXAMIDE
- CAS Number:321534-62-7
- Molecular formula:C17H14N4O4
- Molecular Weight:338.32
Yield:-
Reaction Conditions:
Stage #1: m-Anisidinewith dmap;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 20; for 0.0833333 h;
Stage #2: C10H7N3O4 in dichloromethane at 20; for 18 h;
Steps:
General procedure for benzanilides or nicotinanilides 3-5, 7a-7e, 8a-8d, 8f-8k, 9a, 9c-9n, 11
To a solution of 1.5 mmol N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC·HCl) and 1 mmol 4-dimethylaminopyridine (DMAP) in 2 ml dichloromethane were added 1.5 mmol 3-substituted aniline and the solution stirred at ambient temperature for 5 min. This solution was then added to 1 mmol of substituted benzoic or nicotinic acid and the solution stirred at ambient temperature for 18 hours. The reaction mixture was filtered through a cartridge filled with 5g SCX/silica gel 2:3, pre-washed with 10 ml methanol and 20 ml dichloromethane, and the reaction product eluted with 50 ml dichloromethane. Evaporation provided the benzanilides or nicotinanilides typically as solids.
References:
Stalder, Henri;Hoener, Marius C.;Norcross, Roger D. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 4,p. 1227 - 1231] Location in patent:supporting information; experimental part