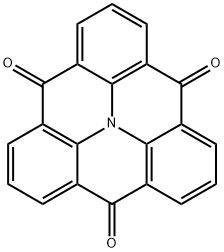
12c-Aza-4H,12H,12cH-dibenzo[cd,mn]pyrene-4,8,12-trione synthesis
- Product Name:12c-Aza-4H,12H,12cH-dibenzo[cd,mn]pyrene-4,8,12-trione
- CAS Number:32081-21-3
- Molecular formula:C21H9NO3
- Molecular Weight:323.3
32081-16-6
2 suppliers
inquiry
![12c-Aza-4H,12H,12cH-dibenzo[cd,mn]pyrene-4,8,12-trione](/CAS/20180808/GIF/32081-21-3.gif)
32081-21-3
1 suppliers
inquiry
Yield:32081-21-3 77%
Reaction Conditions:
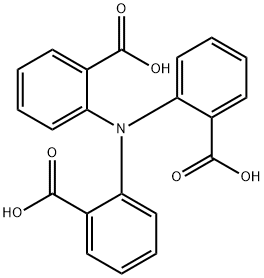
Stage #1: 2,2',2''-nitrilotribenzoic acidwith thionyl chloride in dichloromethane;N,N-dimethyl-formamide at 45; for 4 h;Inert atmosphere;
Stage #2: with anhydrous tin tetrachloride in dichloromethane;N,N-dimethyl-formamide at 45; for 12 h;Inert atmosphere;
Steps:
1 Compound TOAT:
Under the protection of nitrogen, add compound 4 (755mg, 2mmol), thionyl chloride (1.3mL, 18mmol) and N,N-dimethylformamide (0.1mL) into a 100mL two-necked flask. 50mL of dichloromethane solvent was heated to reflux for 4h. Then, tin tetrachloride (2.1 mL, 18 mmol) was added to continue the reflux reaction for 12 hours. The reaction was completely cooled to room temperature, the equivalent of sodium hydroxide aqueous solution was added, and a yellow solid was obtained by filtration. After further purification in dichloromethane/methanol (1:1), 491 mg of yellow solid was obtained with a yield of 77%.
References:
CN111303150,2020,A Location in patent:Paragraph 0045-0046; 0052-0053