
4,5-dihydro-3-methyl-5-oxo-1H-pyrazole-1-acetic acid synthesis
- Product Name:4,5-dihydro-3-methyl-5-oxo-1H-pyrazole-1-acetic acid
- CAS Number:30979-39-6
- Molecular formula:C6H8N2O3
- Molecular Weight:156.14

30979-40-9
4 suppliers
inquiry

30979-39-6
13 suppliers
$302.00/1g
Yield:30979-39-6 90%
Reaction Conditions:
with water;lithium hydroxide for 2 h;Cooling with ice;
Steps:
1.3
Intermediate 1 (5 mmol) and 5 mL of water were stirred uniformly, LiOH (8 mmol) aqueous solution was added dropwise to it under ice bath, HCl (10 mmol) solution was added dropwise to the solution after overnight stirring for neutralization, and stirringwas continued for 2 hours until the reaction was completed.The water solvent was removed by low-pressure distillation, the crude product was prepared by reverse phase liquid phase, andthe eluent was pure water to obtain a white block solid intermediate 2 with a yield of 90%.
References:
CN113214673,2021,A Location in patent:Paragraph 0067; 0075-0078
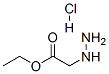
6945-92-2
207 suppliers
$6.00/1g

30979-39-6
13 suppliers
$302.00/1g
