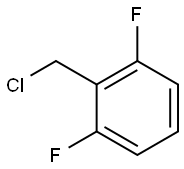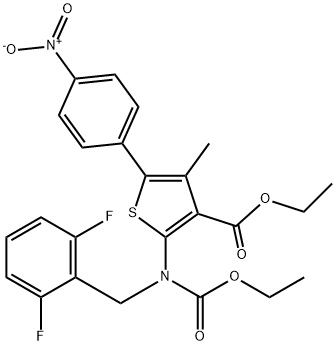
ethyl 2-((2,6-difluorobenzyl)(ethoxycarbonyl)amino)-4-methyl-5-(4-nitrophenyl)thiophene-3-carboxylate synthesis
- Product Name:ethyl 2-((2,6-difluorobenzyl)(ethoxycarbonyl)amino)-4-methyl-5-(4-nitrophenyl)thiophene-3-carboxylate
- CAS Number:308831-94-9
- Molecular formula:C24H22F2N2O6S
- Molecular Weight:504.5
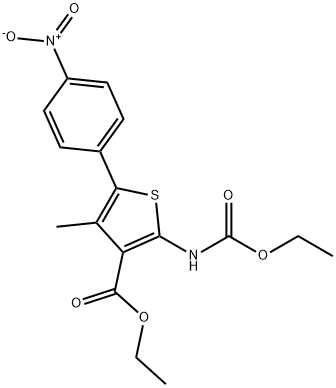
308831-93-8
75 suppliers
inquiry
85118-00-9
335 suppliers
$6.00/1g

308831-94-9
122 suppliers
inquiry
Yield:308831-94-9 88.6%
Reaction Conditions:
with potassium carbonate;potassium iodide in N,N-dimethyl-formamide at 25; for 16 h;
Steps:
4.1.2. ethyl 2-((2,6-difluorobenzyl) (ethoxycarbonyl)amino)-4-methyl-5-(4-nitrophenyl) thiophene-3-carboxylate (6a)
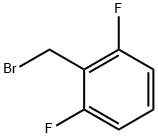
KI (51.91 g, 312.71 mmol, 1.1 eq) and K2CO3 (43.22 g, 312.71 mmol,1.1 eq) were added to a solution of compound 5 (107.57 g, 284.28mmol, 1 eq) in DMF (3400 mL) A solution of 2,6-Difluorobenzyl bromide(55.46 g, 341.14 mmol, 1.2 eq) in DMF (200 mL) was then added to themixture. The mixture was then stirred at 25 C for 16 h. LCMS showedthat the starting material was consumed completely and one peak withdesired MS was detected. The mixture was poured into water (700 mL)and extracted with EtOAc (300 mL × 2). The combined organic layerwas washed with water (500 mL), brine (500 mL), dried over anhydrousNa2SO4, and concentrated under reduced pressure. The residue wassuspended in 600 ml of methyl-tert-butyl ether, and stirred at 25 C for30 min. After filtration, the filter cake was washed by 100 mL of methyltert-butyl ether and dried under vacuum to afford 6a (127 g, 251.73mmol, 88.6 yield) as a yellow solid. MS m/z (ESI): 505[M+H]+. 1H NMR(400 MHz, CDCl3) 1.20 (s, 2H), 1.33 (t, J = 6.80 Hz, 4H), 2.40 (s, 3H),4.18-4.34 (m, 4H), 4.98 (s, 2H), 6.86 (t, J = 8.00 Hz, 2H), 7.22-7.31 (m,1H), 7.52 (d, J = 8.80 Hz, 2H), 8.25-8.30 (m, 2H).
References:
Zou, Fangxia;Wang, Yao;Yu, Dawei;Liu, Chunjiao;Lu, Jing;Zhao, Min;Ma, Mingxu;Wang, Wenyan;Jiang, Wanglin;Gao, Yonglin;Zhang, Rui;Zhang, Jianzhao;Ye, Liang;Tian, Jingwei [European Journal of Medicinal Chemistry,2022,vol. 242,art. no. 114679]
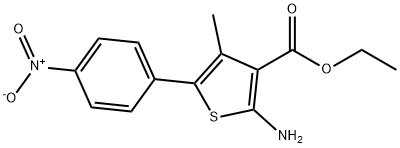
174072-89-0
127 suppliers
inquiry

308831-94-9
122 suppliers
inquiry
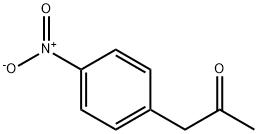
5332-96-7
139 suppliers
$38.79/1g

308831-94-9
122 suppliers
inquiry
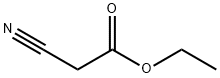
105-56-6
454 suppliers
$10.00/5ml

308831-94-9
122 suppliers
inquiry