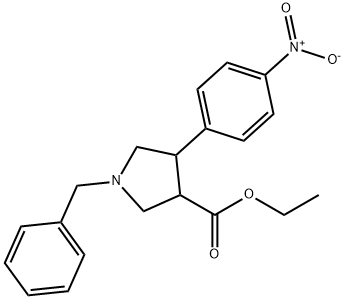
1-BENZYL-4-(4-NITRO-PHENYL)-PYRROLIDINE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:1-BENZYL-4-(4-NITRO-PHENYL)-PYRROLIDINE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:306305-35-1
- Molecular formula:C20H22N2O4
- Molecular Weight:354.4

93102-06-8
2 suppliers
inquiry

306305-35-1
19 suppliers
inquiry
Yield:-
Reaction Conditions:
with oxalic acid;sodium carbonate;trifluoroacetic acid in ethanol;dichloromethane;water;
Steps:
R.6 1-Benzyl-4-(4-nitro-phenyl)-pyrrolidine-3-carboxylic acid ethyl ester
REFERENCE EXAMPLE 6 1-Benzyl-4-(4-nitro-phenyl)-pyrrolidine-3-carboxylic acid ethyl ester A stirred solution of 4-nitroethylcinamate (10 g) in dichloromethane (200 ml), at 23° C., was treated with N-(butoxymethyl)-N-(trimethylsilylmethyl)benzylamine (10 g, prepared as described in Tetrahedron Letters, 1996, 37(43), page 7743-7744) followed immediately by treatment with trifluoroacetic acid (0.25 ml). After stirring at 25° for 20 hours a further aliquot of trifluoroacetic acid (0.5 ml) was added and stirring was continued for a further 20 hours. The reaction mixture was cooled to 5° C., then treated with sodium carbonate (5 g) and then filtered. The filtrate was concentrated under reduced pressure (40° C. and 2.7 kPa) and then treated with anhydrous ethanol (250 ml) followed by oxalic acid (4 g). The mixture was triturated for 10 minutes then stood at 25° C. for 20 hours. The resulting white crystalline solid was filtered then washed twice with ethanol (10 ml), then dried, then treated water (600 ml). The pH of the mixture was adjusted to 6-7 by addition of potassium bicarbonate (10 g) during 30 minutes then extracted with ethyl acetate (400 ml). The organic extract was dried over magnesium sulphate then evaporated (40° C. and 2.7 kPa) affording the title compound (9.36 g) as an orange oil. 1H-NMR [400 MHz, (CD3)2SO]: 671.14 (t, J=7 Hz, 3H); 2.63 (m, 1H); 2.85 (m, 1H); 2.99 (m, 2H); 3.14 (m, 1H); 3.66 (AB system, J=13 Hz, 2H); 3.65 to 3.80 (m, 1H); 4.07 (m, 2H); 7.20 to 7.40 (m, 5H); 7.63 (d, J=8.5 Hz, 2H); 8.18 (d, J=8.5 Hz, 2H). MS (EI): 354[M]+.
References:
US2002/94994,2002,A1