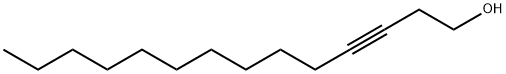
3-TETRADECYN-1-OL synthesis
- Product Name:3-TETRADECYN-1-OL
- CAS Number:55182-74-6
- Molecular formula:C14H26O
- Molecular Weight:210.36
Yield: 75.4%
Reaction Conditions:
Stage #1:3-Butyn-1-ol with ethyl bromide;magnesium in tetrahydrofuran at 5 - 20; for 5.66667 h;Inert atmosphere;
Stage #2: with copper(l) iodide in tetrahydrofuran at 20; for 0.25 h;
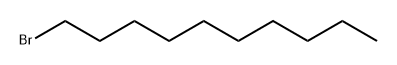
Stage #3:1-bromo dodecaneFurther stages;
Steps:
1.1; 2.1 Preparation of 3-tetradecynyl alcohol-1
Installed with a stirrer, reflux condenser,In a 500 mL four-neck round bottom flask with a constant pressure dropping funnel,Put 65mL of anhydrous tetrahydrofuran and 6.2g of magnesium chips, slowly add 35.2g of a mixture of ethyl bromide and 24mL of tetrahydrofuran under nitrogen protection and stirring, initiate reaction by slight heat, then cool down to 5 ° C, continue to drop Dissolve the magnesium chips, and react at room temperature for 2 hours after the addition.A gray suspension was obtained, and a mixture of 10.22 g of 3-butynyl alcohol-1 and 8 mL of tetrahydrofuran was added dropwise thereto, and the mixture was added in 40 minutes, and the reaction was continued at room temperature for 3 hours, and the temperature was lowered to 0 ° C.0.4 g of freshly prepared cuprous chloride was added and stirred for 15 min. The suspension turned from ash to yellow.Then, a mixture of 20.3 g of 1-bromo-decane and 14 mL of tetrahydrofuran was added dropwise.After the addition, reflux at 65 ~ 70 ° C for 16h,Add 0.2g of cuprous chloride and reflux for 16h.The reaction solution turned dark brown, and 140 mL of a 1 mol/L sulfuric acid solution was slowly added.The reaction liquid was extracted three times with diethyl ether, 100 mL of diethyl ether each time, and the extract was washed successively with 60 mL of saturated sodium carbonate, saturated brine and distilled water, and dried over anhydrous sodium sulfate overnight.Filter by suction and concentrate the filtrate.Distilled under reduced pressure to obtain 12.1 g of a yellow liquid.The yield was 75.4%.
References:
Beijing Forestry University;Zong Shixiang;Luo Youqing;Zhang Jintong;Ren Lili;Liu Jinlong;Tao Jing;Huang Jiandong CN110028405, 2019, A Location in patent:Paragraph 0033; 0035; 0036; 0045-0047
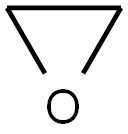
75-21-8
236 suppliers
$33.29/crm44609
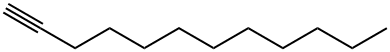
765-03-7
106 suppliers
$33.00/5g

55182-74-6
17 suppliers
inquiry