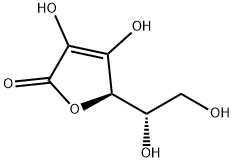
3-O-Ethyl-L-ascorbic acid synthesis
- Product Name:3-O-Ethyl-L-ascorbic acid
- CAS Number:86404-04-8
- Molecular formula:C8H12O6
- Molecular Weight:204.18
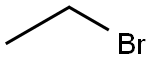
A single-step synthesis of 3-O-ethyl-L-ascorbic acid was performed without the induction of protecting groups. Sodium L-ascorbate reacted with ethyl bromide in DMSO to give 3-O-ethylascorbic acid in a yield of 51.0%.
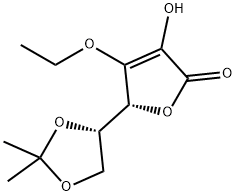
86404-03-7
1 suppliers
inquiry

86404-04-8
656 suppliers
$6.00/1g
Yield:86404-04-8 98%
Reaction Conditions:
with hydrogenchloride in methanol;water at 60; for 3 h;
Steps:
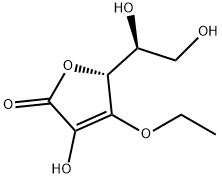
10 Example 10 3-O-Ethyl-5,6-O-isopropylidene ascorbic acid deprotection:
10 g of 3-O-ethyl-5,6-O-isopropylidene ascorbic acid was dissolved in 100 ml of methanol, 5 ml (50% by volume) of hydrochloric acid was added, and the mixture was heated to 60 ° C for 3 hours to react.After neutralizing with sodium bicarbonate and adding ethyl acetate to extract and crystallize, 8.2 g of 3-O-ethyl ascorbic acid was obtained with a yield of 98%.
References:
CN110343096,2019,A Location in patent:Paragraph 0043; 0044
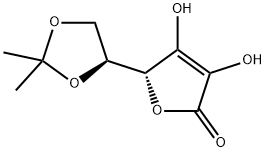
15042-01-0
160 suppliers
$18.00/1g

86404-04-8
656 suppliers
$6.00/1g