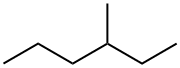
3-METHYLHEXANE synthesis
- Product Name:3-METHYLHEXANE
- CAS Number:589-34-4
- Molecular formula:C7H16
- Molecular Weight:100.2

67-56-1
760 suppliers
$7.29/5ml-f
107-83-5
228 suppliers
$10.00/1g

108-08-7
76 suppliers
$47.29/2g
591-76-4
72 suppliers
$85.00/5mL

565-59-3
83 suppliers
$44.20/5g

589-34-4
76 suppliers
$95.79/5g
75-28-5
125 suppliers
$40.00/1g
78-78-4
273 suppliers
$20.00/25ML
96-14-0
106 suppliers
$22.19/5ml
79-29-8
138 suppliers
$20.00/1g
464-06-2
92 suppliers
$23.50/1g
Yield:-
Reaction Conditions:
indium (III) iodide at 180; for 0.5 h;
Steps:
23
In order to demonstrate that InI3 catalyzed alkane homologation can be used to homologate C6 alkanes other than 2,3-dimethylbutane, a reaction was performed using 2-methylpentane as the starting alkane. The reaction was performed using 6.2 mmol of MeOH, 6.2 mmol of 2-methylpentane, 4.13 mmol of InI3 and 0.367 mmol of adamantane. A similar procedure to that described above for 2,3-dimethylbutane was used and the reaction was heated at 180 0C for 30 minutes. GC analysis indicated that at the end of the reaction 27.4 mg of 2,3-dimethylpentane was formed and 267 mg of 2-methylpentane were recovered. A breakdown of the product distribution is shown in Table 8.Table 8: Products from the homologation of 2-methylpentane with MeOH.Product Yield (mg)Iso-butane 23.5Iso-pentane 10.72,3-dimethylbutane 1.762-methylpentane 2673-methylpentane 73.02,4-dimethypentane 12.1Triptane 5.032-methylhexane 5.252,3-dimethylpentane 27.43-methylhexane 8.48Cs alkanes 17.3[0088] The reaction is presumed to initially result in the methylation of 2- methylpentane to 2,3-dimethylpentane. The mechanism is proposed to be analogous to that described above for 2,3-dimethylbutane; initial abstraction of a hydride from 2- methylpentane to form the tertiary 2-methylpentyl carbocation, deprotonation to give 2-methyl-2-pentene, methylation to give the most substituted carbocation, in this case the tertiary 2, 3-dimethylpentyl carbocation, which subsequently picks up a hydride to form 2,3-dimethylpentane. Under the reaction conditions, 2,3- dimethylpentane is unstable and undergoes both isomerization and cracking reactions to form some of the other products observed. Isomerization of 2,3-
References:
CALIFORNIA INSTITUTE OF TECHNOLOGY;BP P.L.C. WO2009/64622, 2009, A2 Location in patent:Page/Page column 27-28
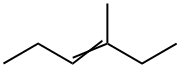
3404-65-7
0 suppliers
inquiry

589-34-4
76 suppliers
$95.79/5g
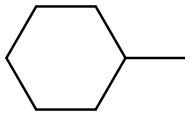
82166-21-0
0 suppliers
inquiry

591-76-4
72 suppliers
$85.00/5mL

589-34-4
76 suppliers
$95.79/5g

75-28-5
125 suppliers
$40.00/1g
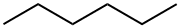
110-54-3
801 suppliers
$20.00/100ml

590-35-2
70 suppliers
$34.00/1mL
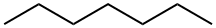
142-82-5
549 suppliers
$16.00/25ML
96-37-7
159 suppliers
$10.00/1g
1638-26-2
32 suppliers
$1320.00/250mg
1640-89-7
40 suppliers
$45.00/10mg
2453-00-1
25 suppliers
inquiry
106-97-8
109 suppliers
$36.00/1mL

4806-62-6
1 suppliers
inquiry

589-34-4
76 suppliers
$95.79/5g
142-82-5
549 suppliers
$16.00/25ML

71-43-2
706 suppliers
$11.19/25ML