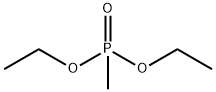
(3-METHYLBENZYL)PHOSPHONIC ACID DIETHYL ESTER synthesis
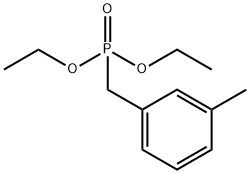
- Product Name:(3-METHYLBENZYL)PHOSPHONIC ACID DIETHYL ESTER
- CAS Number:63909-50-2
- Molecular formula:C12H19O3P
- Molecular Weight:242.25
Yield:-
Reaction Conditions:
for 10 h;Reflux;
Steps:
1
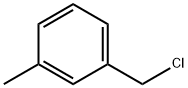
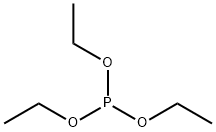
To 29.0 g (207 mmol) of α-chloro-m-xylene, 37.8 g (228 mmol) of triethyl phosphite was added. The mixture was heated under reflux for 10 hours to obtain 52.3 g of a colorless liquid. Then, 47.3 g (187 mmol) of the colorless liquid was dissolved in 300 ml of N,N,-dimethylformamide (DMF), and 27.6 g (197 mmol) of p-chlorobenzaldehyde was added thereto. Under a nitrogen atmosphere, 23.1 g (206 mmol) of potassium-tert-butoxide was added thereto, followed by an additional stirring for 2 hours. The resulting mixture was neutralized with water and hydrochloric acid, and extracted with toluene. The toluene layer was washed with water, then concentrated, and subjected to a recrystallization operation. As a result, 36.6 g of a stilbene derivative was obtained. The yield was 85.6%.Under a nitrogen atmosphere, 34.5 g (150 mmol) of the obtained stilbene derivative, 13.5 g (141 mmol) of sodium tert-butoxide, 1.1 g (47 mmol) of lithium amide, 172 mg (0.47 mmol) of [PdCl(allyl)]2, and 441 mg (1.13 mmol) of 1,1-diphenyl-2-(dicyclohexylphosphino)propene were added into 160 ml of xylene, and the mixture was heated to 100° C. After stirring for 5 hours, water was added thereto, and further toluene was added thereto. Then, the organic layer was separated. The organic layer was washed with water, and then concentrated. The residue was subjected to silica gel column chromatography to remove impurities, and further subjected to a recrystallization operation. As a result, 24.0 g of a yellow crystal was obtained. The yield was 86%. An HPLC analysis showed that the ratio of the ttt isomer was 100%. Moreover, 1H NMR showed that the cis-trans ratio of the double bonds was 0:100.1H NMR (CDCl3): δ; 2.38 (s, 9H), 7.00 (d, J=16.3 Hz, 3H), 7.07 (d, J=7.4 Hz, 3H), 7.07 (d, J=16.3 Hz, 3H), 7.11 (d, J=8.6 Hz, 6H), 7.24 (t, J=7.5 Hz, 3H), 7.31 (d, J=8.1 Hz, 3H), 7.33 (s, 3H), 7.42 (d, J=8.6 Hz, 6H).MS (m/Z) 593mp 98-99° C.
References:
US2012/22293,2012,A1 Location in patent:Page/Page column 10
683-08-9
125 suppliers
$29.00/1g

63909-50-2
47 suppliers
$9.00/1g